| dc.contributor.author | Desplan, C | fr_FR |
| dc.date.accessioned | 2012-07-11T08:41:05Z | |
| dc.date.available | 2012-07-11T08:41:05Z | |
| dc.date.issued | 1997 | fr_FR |
| dc.identifier.citation | Desplan, C, Fonction des gènes Pax : synergie de liaison à l'ADN entre le domaine paired et l'homéodomaine., Med Sci (Paris), 1997, Vol. 13, N° 2; p.147-55 | fr_FR |
| dc.identifier.issn | 1958-5381 | fr_FR |
| dc.identifier.uri | http://hdl.handle.net/10608/330 | |
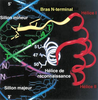
| dc.description.abstract | Les gènes du développement Pax, codant pour des régulateurs
de transcription, sont caractérisés par la présence
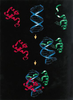
d’un domaine paired (PD) capable de se lier à l’ADN.
Celui-ci comporte deux motifs hélice-tour-hélice, appelés
PAI et RED. Certaines protéines Pax possèdent également
un troisième domaine de liaison à l’ADN, l’homéodomaine
(HD). Des études in vitro et in vivo chez la drosophile pourraient
expliquer, par une combinatoire de ces divers
domaines de liaison à l’ADN, la très grande spécificité fonctionnelle
des protéines Pax, alors que ces domaines reconnaissent
des séquences d’ADN très semblables. | fr |
| dc.description.abstract | The Pax genes encode a set of transcriptional regulators involved in several developmental processes. They are characterized by the presence of the paired domain (PD), a DNA binding domain composed of two Helix-Turn-Helix (HTH) motifs, the PAI and RED domains. Some Pax proteins contain a third HTH DNA binding motif, the homeodomain (HD). Since all PDs recognize highly related DNA sites, and since all HDs recognize a common TAAT sequence, it has been difficult to understand how these proteins achieve their functional specificity. Here, we describe how different Pax proteins use multiple combinations of their DNA binding motifs to target different promoters. In vitro, the Drosophila paired protein can bind either through its PAI domain, through its HD via cooperative dimerization, or through both domains. In vivo, using a transgenic rescue assay, we show that prd function requires the synergistic action of both the PAI domain and the HD on abutted PD and HD sites. Surprisingly, the RED domain, although conserved, can be deleted without loss of viability. This is in contrast to other Pax proteins that require both PAI and RED domains. Furthermore, specific isoforms of Pax6 as well as a new Pax protein, Lune, may rely on the RED domain alone. Finally, we propose that Pax6 may also act through its HD alone on a series of highly conserved palindromic TAAT sites found in all rhodopsin promoters. This may represent the ancient function of this master regulator of eye development before it acquired a PD. [References: 39] | en |
| dc.language.iso | fr | fr_FR |
| dc.publisher | Masson, Paris | fr_FR |
| dc.rights | Article en libre accès | fr |
| dc.rights | Médecine/Sciences - Inserm - SRMS | fr |
| dc.source | M/S. Médecine sciences [revue papier, ISSN : 0767-0974], 1997, Vol. 13, N° 2; p.147-55 | fr_FR |
| dc.title | Fonction des gènes Pax : synergie de liaison à l'ADN entre le domaine paired et l'homéodomaine. | fr |
| dc.title.alternative | Synergism in DNA binding of paired and homeo domains in Pax protein : French-speaking scientists in the United States | fr_FR |
| dc.type | Article | fr_FR |
| dc.contributor.affiliation | Howard Hughes Medical Institute, The Rockefeller University, 1230 York Avenue, New York, NY 10021-6399, United States | - |
| dc.identifier.doi | 10.4267/10608/330 | |