| dc.contributor.author | Fattoum, A | fr_FR |
| dc.date.accessioned | 2012-07-11T08:41:52Z | |
| dc.date.available | 2012-07-11T08:41:52Z | |
| dc.date.issued | 1997 | fr_FR |
| dc.identifier.citation | Fattoum, A, Régulation de la contraction du muscle lisse, Med Sci (Paris), 1997, Vol. 13, N° 6-7; p.777-89 | fr_FR |
| dc.identifier.issn | 1958-5381 | fr_FR |
| dc.identifier.uri | http://hdl.handle.net/10608/462 | |
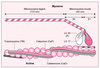
| dc.description.abstract | Il est établi depuis longtemps que la phosphorylation spécifique,
dépendante du complexe Ca2+-calmoduline, des
chaînes légères régulatrices (LC20) de la myosine par la
kinase correspondante (MLCK) représente un événement
initiateur capital dans le développement de la force
contractile du muscle lisse. Actuellement, on démontre que
le filament fin naturel possède également la propriété de
moduler la vitesse de raccourcissement de la fibre musculaire
en agissant sur le complexe acto-myosine par l’intermédiaire
de protéines de découverte récente, fortement
liées à l’actine, telles que la caldesmone et la calponine.
L’intervention alternée des deux systèmes de régulation,
l’un au niveau des filaments épais et l’autre au niveau des
filaments fins, pourrait expliquer certaines propriétés du
latch-bridge, phénomène caractéristique du maintien d’un
état contracté en phase tonique, propre au muscle lisse, et,
plus particulièrement, au tissu vasculaire, observé lors de
la baisse du niveau de phosphorylation de la myosine. | fr |
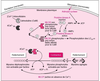
| dc.description.abstract | Muscle contraction is driven by a cyclical interaction between the globular head domain of myosin and the actin filament, coupled to the breakdown of ATP. In vertebrate smooth muscle, it is generally accepted that actomyosin ATPase and consequently tension generation are regulated primarily by the reversible phosphorylation of the 20 kDa myosin light chain. However, current studies have suggested that the simple on/off system based on myosin phosphorylation is not sufficient to explain all the aspects of the contractile activity of smooth muscle, specifically as concerns the tonic responses in the slow cycling, termed 'latch-bridge', characteristic of sustained isometric force production. Today, it is thought that caldesmon and calponin, two thin-filament proteins discovered in the 1980's, are the two major elements required in the mechanism of Ca2+-dependent tension maintenance without detectable elevation in phosphorylation, especially in vascular smooth tissue. Both of these proteins can bind to all the major proteins that make up the actomyosin system (actin, myosin, tropomyosin, etc.) and inhibit the actin-activated Mg-ATPase activity of myosin. This inhibitory action can be reversed upon their interaction with EF-hand Ca-dependent proteins such as calmodulin, caltropin and S-100, or through changes in their phosphorylation state. Further, investigations of the properties of caldesmon and calponin in vitro and in vivo have led to the conclusion that these proteins are likely to be involved in an actin-linked Ca2+-sensitive secondaryregulatory process operating in the smooth muscle contractility mechanism, independent of the well established myosin phosphorylation system. The interplay between the thick and thin filament-based regulatory systems may explain some of the properties unique to smooth muscle cells, in particular the phenomena of the latch state. [References: 47] | en |
| dc.language.iso | fr | fr_FR |
| dc.publisher | Masson Périodiques, Paris | fr_FR |
| dc.rights | Article en libre accès | fr |
| dc.rights | Médecine/Sciences - Inserm - SRMS | fr |
| dc.source | M/S. Médecine sciences [revue papier, ISSN : 0767-0974], 1997, Vol. 13, N° 6-7; p.777-89 | fr_FR |
| dc.title | Régulation de la contraction du muscle lisse | fr |
| dc.title.alternative | Contractile events in smooth muscle cells | fr_FR |
| dc.type | Article | fr_FR |
| dc.contributor.affiliation | Crns-UPR 9008, Inserm U.249, universite de Montpellier I, route de Mende, BP 5051, 34033 Montpellier, France | - |
| dc.identifier.doi | 10.4267/10608/462 | |