| |
| Med Sci (Paris). 36(3): 285–288. doi: 10.1051/medsci/2020044.Étude du système de gestion de l’information
(biobank information management system) pour les
annotations clinico-biologiques des biobanques Caroline Le Queau,1* Wendy Ann Phillips,1** and Apoorva Srinivasan1*** 1MSc biobanks and complex data management, Université Côte
d’Azur, Centre hospitalier universitaire de Nice, Hôpital
Pasteur, Biobanque
BB-0033-00025, Nice, France MeSH keywords: Biobanques, Recherche biomédicale, Commerce, Curation de données, Bases de données factuelles, Gestion de l'information en santé, Humains, Gestion de l'information, Innovation organisationnelle, Flux de travaux, économie, organisation et administration, normes, méthodes |
Une partie des étudiants de ce master n’écrivant pas en français, mais étant très
intéressés par cette volonté de médecine/sciences de « donner la parole
à nos jeunes pousses », se sont proposés pour effectuer le même travail que leurs
homologues francophones de ce même master. Exceptionnellement, m/s leur a donné l’occasion d’effectuer cet exercice
en anglais, tout en leur souhaitant un bon apprentissage de notre langue auprès de leurs
enseignants et de leurs collègues étudiants. The biobank information management system (BIMS) is an essential tool that gathers the
data of a laboratory information management system (LIMS), commonly used in laboratories
to track samples and all other informatics systems involving information related to
patients and samples (e.g., electronic medical report, freezer temperature monitoring
systems, etc.). Biologists and clinicians are increasing their efforts to unravel the
biological pathways that underlie human diseases. The use of omics analyses (e.g.,
genomics, transcriptomics, proteomics) has generated extremely large data sets. The
analysis and integration of these data, as well as the constitution of database
platforms, represent an ongoing challenge. This article aims to introduce the
construction of BIMS in relation to a biobank workflow, the multiple solutions that
currently exist on the market, as well as its importance in public and private
research. |
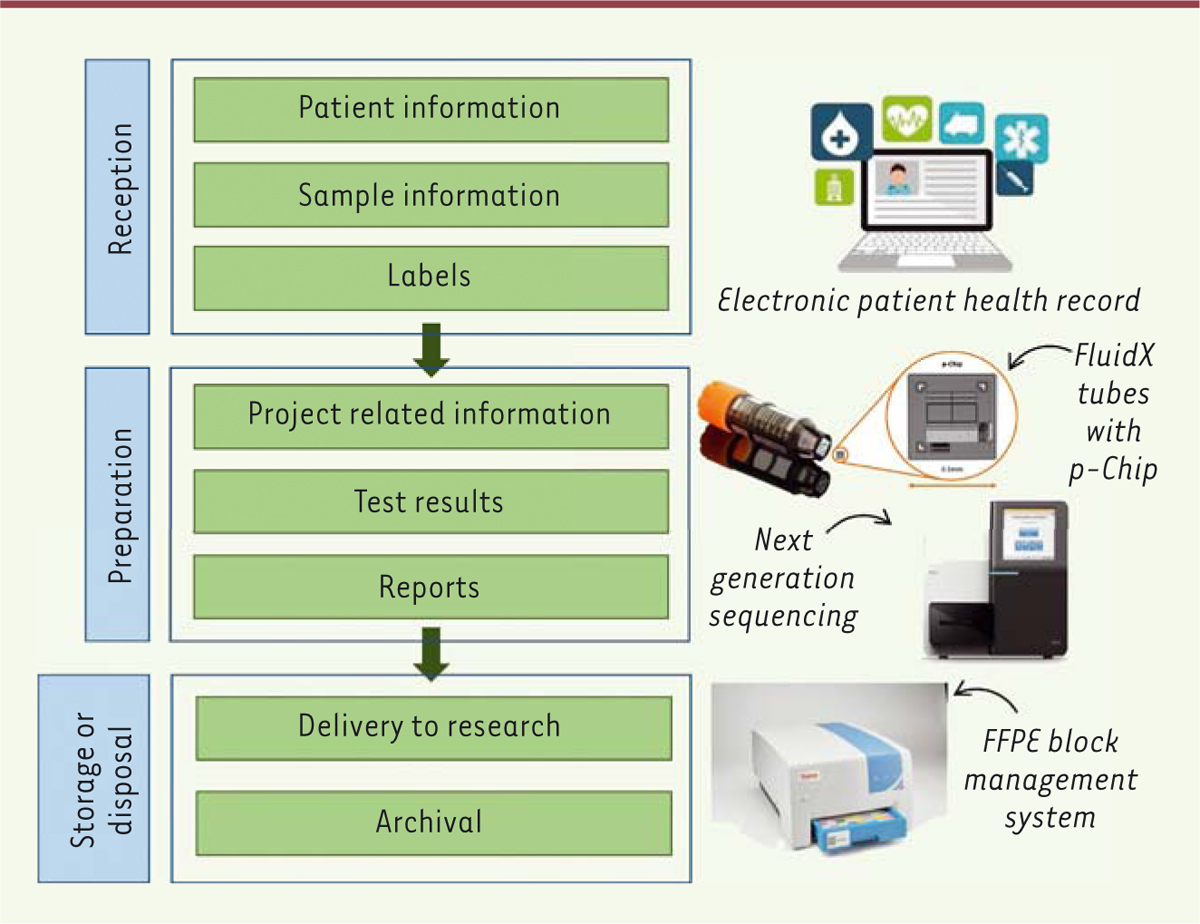
A laboratory information management system (LIMS) is a software allowing the whole
management of samples workflow, including their reception, preparation, storage and
delivery (or the delivery of results of external tests performed on these samples)
(Figure 1). This type
of software facilitates the standardization of tests and procedures, and provides
accurate controls of processes [1].
 | Figure 1. Representation of a typical biobanking data workflow with examples of
associated technologies. In blue are the main steps related to sample
flow, and in green the actions related to data. On the right are
examples of devices that provide data about the sample. The biobank is
keeping in its record information related to the samples for
traceability, for example data delivered to research will remain in the
biobank archives. |
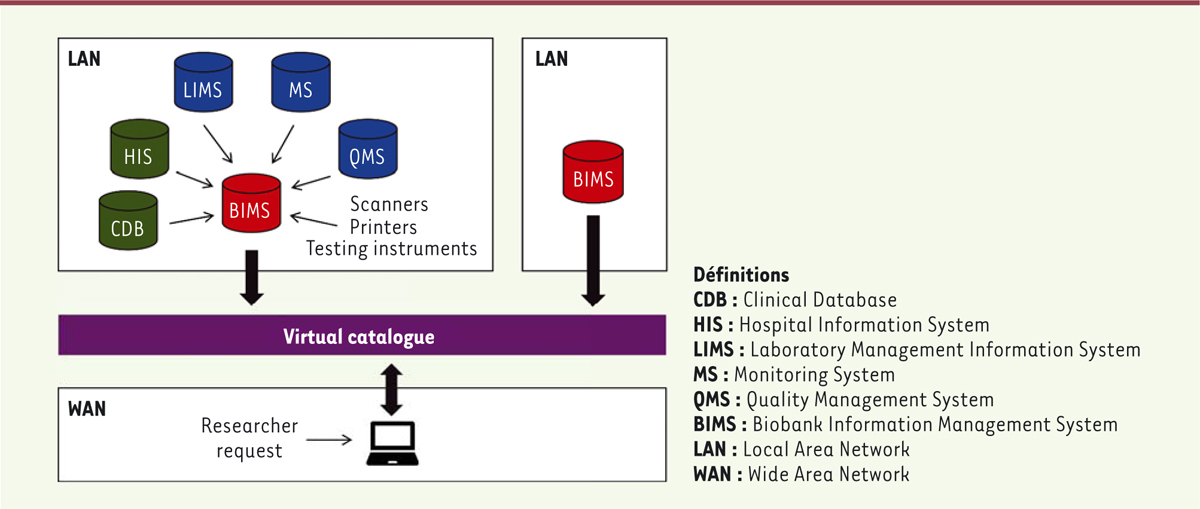
By contrast to a LIMS, a BIMS integrates different pieces of information concerning
both sample characteristics and clinical data related to the patients (including
informed consent), as shown on Figure
2. Hospital information systems (HIS) and clinical database
(CDB), which are sources of clinical data concerning the patients, are governed by
strict regulations especially regarding anonymity [2]. These databases are either accessible from
hospital-owned servers within the biobank or connected to the BIMS through specific
informatics languages [3] in
accordance with European general data protection regulation 2016/679 [4] and as recommended by the
Health Level 7 international standards for transfer of data between software
applications.
 | Figure 2. Representation of a BIMS. Various databases are integrated in the BIMS,
to preserve the confidentiality of informations. The software is hosted
in the LAN, a network that links computers within a restricted area.
Information from the BIMS can be extracted and displayed in a virtual
catalogue, available to researchers. This system allows multisite
biobanks as the catalogue can be linked to more than one BIMS. |
BIMS can also incorporate information from monitoring systems (MS), such as the
freezer temperature or liquid nitrogen level in tanks. The quality management system
(QMS) is often saved on separated software. However, preparation procedures or
documents related to corrective actions in case of non-conformity can be available
through the BIMS. Finally, it can integrate interoperability with test instruments,
such as sequencing machines, in order to suppress the step of manually adding data
to the software. As BIMS store sensitive and confidential data, they are usually localised in local
area networks (LAN), a computer network that interconnects computers within a
restricted area (e.g., hospital or enterprise). Nevertheless, researchers can search
sample availability, from a virtual catalogue on the internet (wide area network),
according to predefined criteria. This catalogue can also gather information from
different sites of a multisite biobank, for example in the case of UK Biobank. The use of BIMS can be varied ; thus, they are usually sold with a certain number of
licenses, i.e. access accounts. As the information can come from different entities
(hospital, external or internal laboratories, biobank) it is crucial that the
biobank controls the access to its BIMS. Limited access can be created so that a
specific member of staff can only enter a specific information. For example,
hospital staff can add barcodes and time of sampling, the clinician can add
patients’ data, and biobanking staff can manage the sample workflow. All requests
regarding the access and use of the BIMS should be discussed prior to the
development of biobanks. Biological or clinical information collected and stored on a BIMS are varied, and the
data format heterogeneous. They are linked together through data models that need to
be thoroughly studied in order to avoid mistakes in analysis. Data registered from
various sources and gathered manually increase the time of entry and the risk of
error, whereas BIMS allows automatic integration, thus reducing potential
mistakes. Therefore, it leads to complexity in terms of software design and storage space. An
efficient BIMS needs to be flexible enough to support from the smallest to the
largest collection of samples, and to manage different stakeholders’ accesses.
Finally, the BIMS design has to take into account the permanent update of the stored
data and the necessity for users to easily have access to these data. |
The growing market surrounding data management does not leave biobanking behind, as
shown by the multiplication of BIMS solutions available. Companies1 such as LabVantage Solutions
Inc., Genohm, Brooks Life Sciences,
Technidata, Modul-bio, and Thermo
Fisher Scientific all developed their own BIMS solution in adequacy
with different customers’ needs [5]. Variability exists in terms of possible interoperability,
personalisation of modules, maintenance efficiency, etc. The cost includes software
initial installation, maintenance and upgrade. It also depends on the number of
sites, licenses and modules needed. Open source of made in-house software also
exists and represents a cheaper alternative. However, this kind of approach requires
an informatics competent staff that can modify the software source code. The quality of a biobank service is assessed by both the number of valuable samples
and the full chain of custody of these samples. Data quality is the core business of
a biobank as samples have limited value in research without accurate and reliable
data. Although a BIMS is not mandatory to obtain accreditation according to the
biobank French norm NFS 96-900, it is a long-term investment that will facilitate
the certification, the management of growing sample amount and the homogeneity of
data. This BIMS solution used to record laboratory and sample data in an electronic system
thus helps to improve the robustness of quality systems and sustainability of
biobanking structures. |
How does a BIMS improve research? BIMS is a highly recommended and valuable management system used by biobanks to
improve and maintain the quality of research results. Prior to clinical trials, clinical annotations such as phenotypic, genotypic, and
environmental information gathered through BIMS can allow the determination of
patient groups, e.g., analysis of patient’s DNA sequences to determine different
groups of patients in order to determine the right concentration of drug to treat
each group [6]. Integration
of the maximum of data related to the patient and the sample could help clinicians
and researchers to discover new therapeutic targets, develop innovative strategies
and drugs. The data may originate from various research studies, small and large, where data
formats and data collection methods vary significantly [6]. Managing data through BIMS provides a unique access, thus
enhancing public availability of clinical and biological annotations (e.g.,
UK biobank). Currently, hospitals produce a large amount of data incoming from daily care that is
not exploited at the maximum capacity [7]. BIMS or data centres are a solution to gather data and use
them for in silico studies, i.e., computer-based studies. Big private pharmaceutical companies are interested in building their own biobank to
support their medical research such as drug development or in vitro
diagnostic tests [8]. Smaller companies can collaborate with public biobanks to have access to rare
samples. Moreover, building a private collection is costly and not valuable if it
only serves one purpose. Instead of building its own collection of formalin fixed
paraffin embedded blocks, (FFPE blocks) from cancer patients, a pharmaceutical
company can make a contract with a biobank to obtain slides and data from those
patients. Thereby both stakeholders can share their expertise. During the different
drug development stages, a lot of samples are required, processed and stored. A
partnership with a biobank allows using their expertise in sample collection and
already existing storing facilities. Nevertheless, pharmaceutical companies aim to
work with top quality biobanks, thus valorising quality, full data set,
traceability, etc., all of which can be provided through BIMS. |
BIMS softwares are complex to build and require knowledge and expertise in the fields
of informatics and biobanking. Currently the main challenge is the lack of
interoperability between existing LIMS and software used in healthcare. The future
of medicine lies in the use of clinical annotations for in silico
studies, i.e., studies performed using computer simulation. Creating a catalogue of
massive data integrating online analysis will allow an easier access to shared data
for all researchers. |
Les auteurs déclarent n’avoir aucun lien d’intérêt concernant les données
publiées dans cet article.
|
Footnotes |
1.
Malm
J,
Fehniger
TE,
Danmyr
P, et al.
Developments in biobanking workflow standardization providing
sample integrity and stability . J
Proteomics.
2013; ; 95 :
:38.–45. 2.
Loi n° 78–17 du 6 janvier 1978 modifiée relative à l’informatique, aux
fichiers et aux libertés. Article 32.
3.
Späth
MB,
Grimson
J. Applying the
archetype approach to the database of a biobank information management
system . Int J Med Inform.
2011; ; 80 :
:205.–226. 4.
Regulation (EU) 2016/679 of the European Parliament and of the Council
of 27 April 2016 on the protection of natural persons with regard to the
processing of personal data and on the free movement of such data, and
repealing Directive 95/46/EC (General Data Protection Regulation).
5.
Orchard-Webb D. 10 Top Laboratory Information Management Systems (LIMS)
for biobanking. Biobanking.com 2018.
6.
Olund
G,
Lindqvist
P,
Litton
JE. BIMS: An
information management system for biobanking in the 21st
century . IBM Systems Journal.
2007; ; 46 :
:171.–182. 7.
Cournau
C.. L’hôpital soigne
ses données médicales . Sciences et Avenir.
2018; ; 861 :
:70.–73. 8.
Begley
CG,
Ellis
LM. Drug
development: Raise standards for preclinical cancer
research . Nature.
2012; ; 483 :
:531.–533. |