| |
| Med Sci (Paris). 34: 20–25. doi: 10.1051/medsci/201834f104.Bone and dental abnormalities as first signs of familial Gardner’s syndrome in a Chinese family: a literature review and a case report Dan Yu,1 Benjamin NG CW,2 Huiyong Zhu,3 Jianhua Liu,4 and Yi Lin5* 1M.S., D.D.S., Attending doctor, Department of Oral and Maxillofacial Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University. 79# Qingchun Road, Hangzhou310003, People’s Republic of China 2B.D.S., Resident, Department of Oral and Maxillofacial Surgery, National Dental Centre Singapore. Second Hospital Avenue, 168938, Singapore 3M.D., Ph.D., Professor, Department of Oral and Maxillofacial Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University. 79# Qingchun Road, Hangzhou310003, People’s Republic of China 4M.D., Ph.D., Professor and Chairman, Department of Oral and Maxillofacial Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University. 79# Qingchun Road, Hangzhou310003, People’s Republic of China 5M.S., D.D.S., Attending doctor, Department of Oral and Maxillofacial Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University. 79# Qingchun Road, Hangzhou310003, People’s Republic of China |
Gardner’s syndrome (GS) is an autosomal dominant disease characterized by the presence of familial adenomatous polyposis (FAP) as well as extraintestinal manifestations such as osteomas, dental anomalies, epidermoid cysts and ocular abnormalities. These intestinal polyps carry a 100% risk of malignant change, so early diagnosis is crucial. As craniofacial osteomas and dental anomalies of GS usually precede gastrointestinal symptoms, otolaryngologists, oral surgeons and dentists play an important role in the diagnosis of GS. GS is extensively reported in literature in the Caucasian race but not in the Mongoloid race. We report a case of a 22-year-old patient with a manifestation of three features of GS - multiple osteomas, soft tissue tumors and dental anomalies in the craniofacial region, with no intestinal polyps at the time of reporting. A family pedigree with our patient as the proband was constructed and revealed 3 consecutive generations in his lineage with GS. Keywords: Familial adenomatous polyposis, Gardner’s syndrome, osteomas |
Gardner’s syndrome (GS) is a subgroup of familial adenomatous polyposis (FAP), comprising approximately 10% of FAP patients [1]. It is a rare autosomal dominant inherited disease characterized by the presence of multiple polyps in the intestine as well as bony, cutaneous, dental, and ocular abnormalities [2], due to mutations of the adenomatous polyposis coli (APC) gene on chromosome 5q21-22. There is almost complete penetrance of the intestinal phenotype but variable penetrance of the extraintestinal features. GS is extensively reported in literature in Caucasians but not in Mongoloids. The incidence of FAP is between 1 in 8,300 and 1 in 14,025 births, affecting both genders equally, with a uniform worldwide distribution [3]. FAP is characterized by adenomatous polyps of the colon and rectum with 100% potential of malignant change. This is a maliciously premalignant disease with polyp(s) showing dysplasia and progressing to malignancy in untreated gene carriers, diagnosed at a median age of 40 years [1]. Carcinoma may develop at any age from the second to the seventh decade [1]. The clinical diagnosis of GS is often difficult due to the great variation in the extra-intestinal clinical features [1]. Some patients have only 1 or 2 abnormalities while others show all or many of the characteristic features [4]. The lesions present themselves in a different chronological order, but typically, cutaneous and bony lesions appear 10 years earlier than intestinal polyposis [5]. As craniofacial bony and dental signs of GS often precede gastrointestinal symptoms [2], otolaryngologists, oral surgeons and dentists may play an important role in the diagnosis of FAP, and may potentially save the lives of these patients and their family. Osteomas are usually located in the jaws and frontal bone. Dental anomalies include impacted teeth, supernumerary teeth, odontoma, and congenitally missing teeth. The simultaneous presence of osteoma(s) with dental anomalies with is highly suggestive of underlying GS [6]. We present a Chinese patient with GS who was the proband in his family, from which further investigations allowed the diagnosis of the disease across 3 generations. |
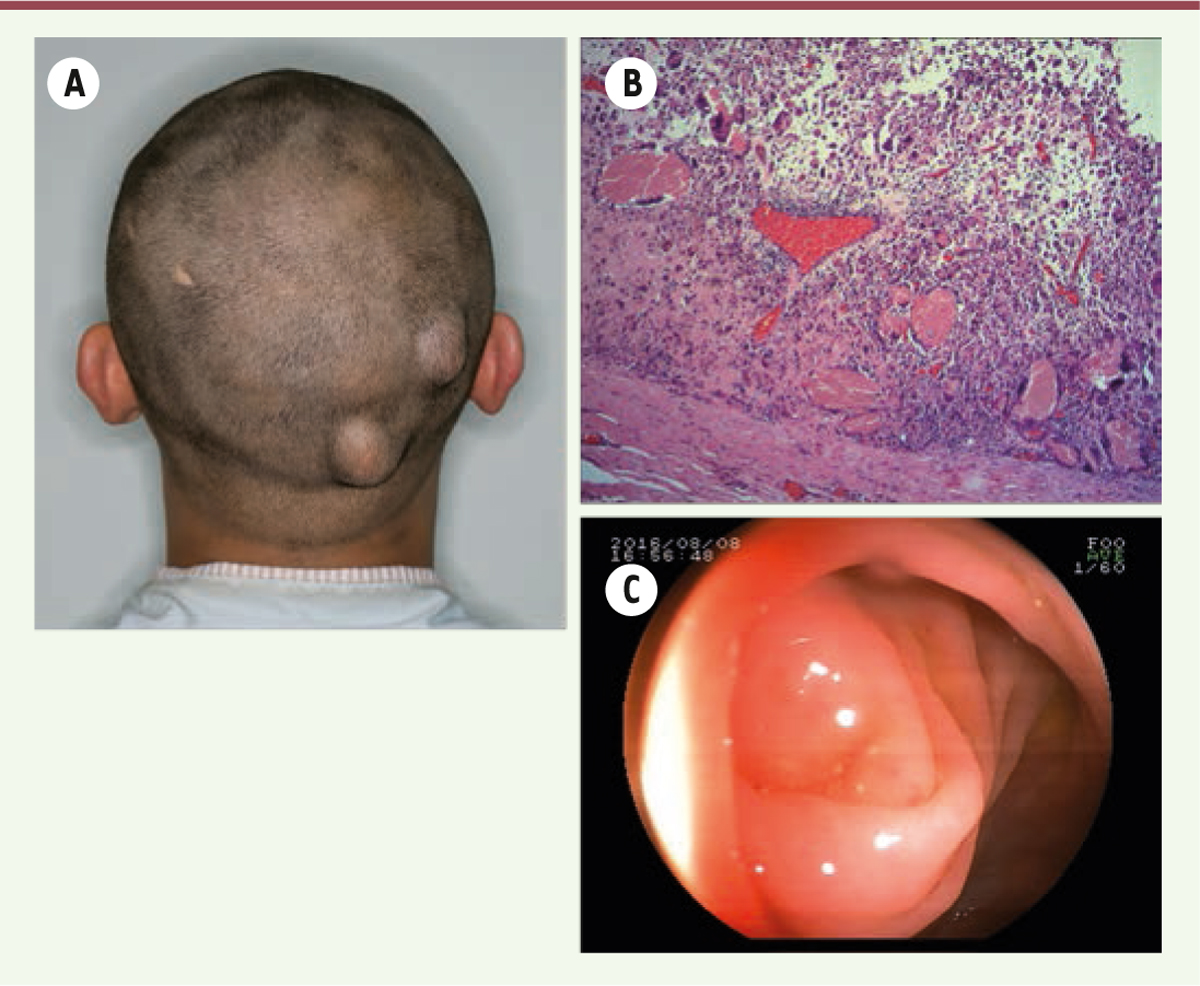
A 22-year-old Chinese man was referred to us for multiple osteomas of the facial bones for the past 7 years, which had increased gradually in size, posing to be a major esthetic problem. A detailed history taking revealed chronic hematochezia and the presence of soft tissue lumps in his scalp (Figure 1A) and scrotum. Further probing elicited that his elder sister and father had multiple bony swelling of the jaws and skull along with colorectal adenomatous polyposis, and his father had undergone colectomy.
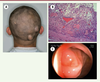 | Figure 1. (A) View of the patient showing two soft tissue lumps in his scalp. (B) Histopathological results revealing the epidermoid cyst with pilomatricoma-like changes (hematoxylin-eosin stain, 5x). (C) A colonoscopy showing normal colonic mucosa. |
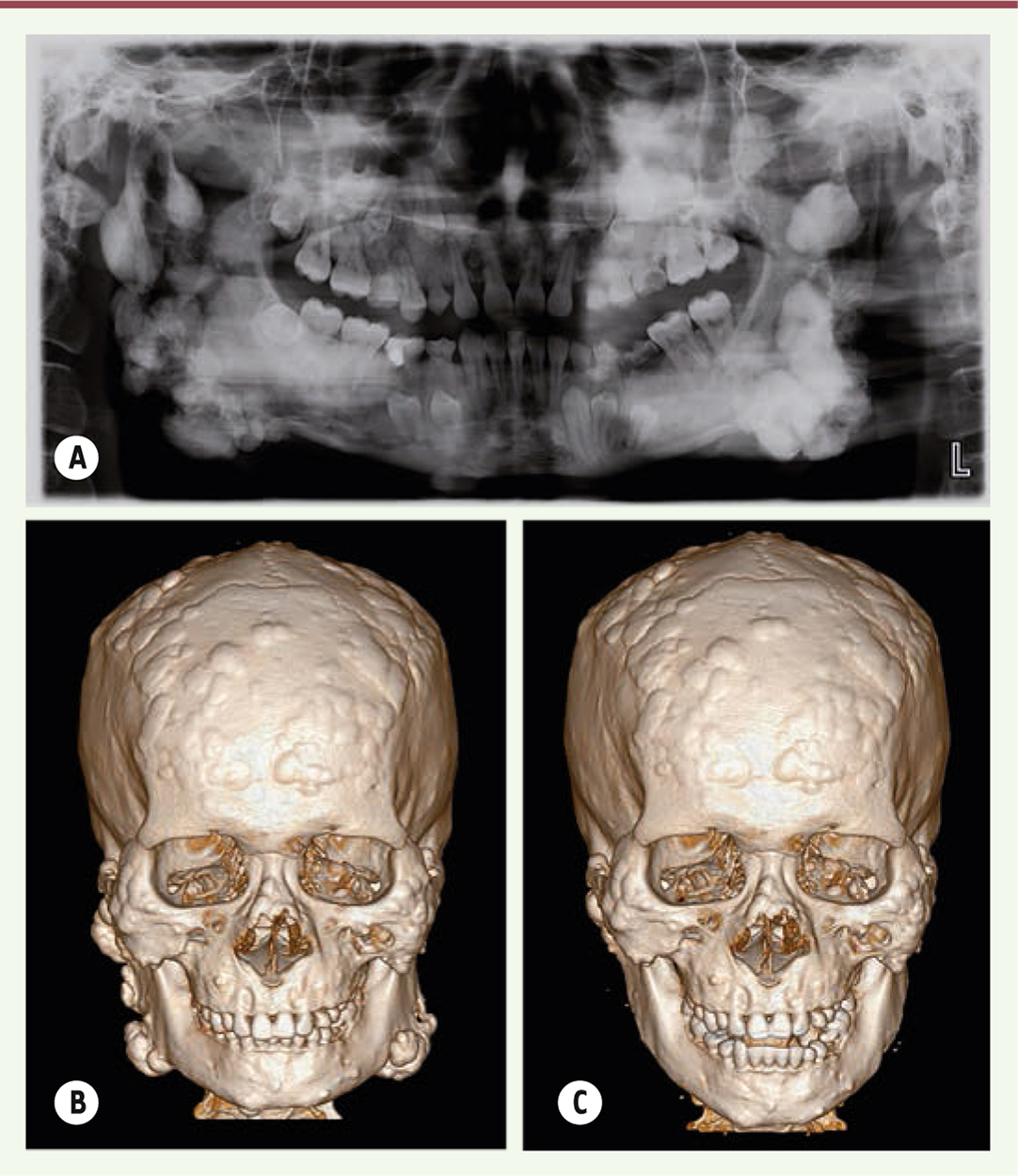
General examination showed multiple nodular formations in the frontal and bilateral mandibular region leading to a facial dysmorphism. On palpation, these tumors were hard, well defined, and immobile. Panoramic radiograph showed multiple radiopaque masses and impacted teeth in the jaws (Figure 2A). The computed tomography (CT) scan showed numerous osteomas of different sizes located on the mandible, as well as all over the calvaria (Figure 2B).
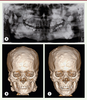 | Figure 2. (A) Panoramic radiograph showing widespread radiopaque lesion and multiple impacted permanent teeth. (B) A preoperative CT view of multiple nodular lumps throughout the jaws and skull surface. (C) A postoperative CT showing a smooth counter line of mandible. |
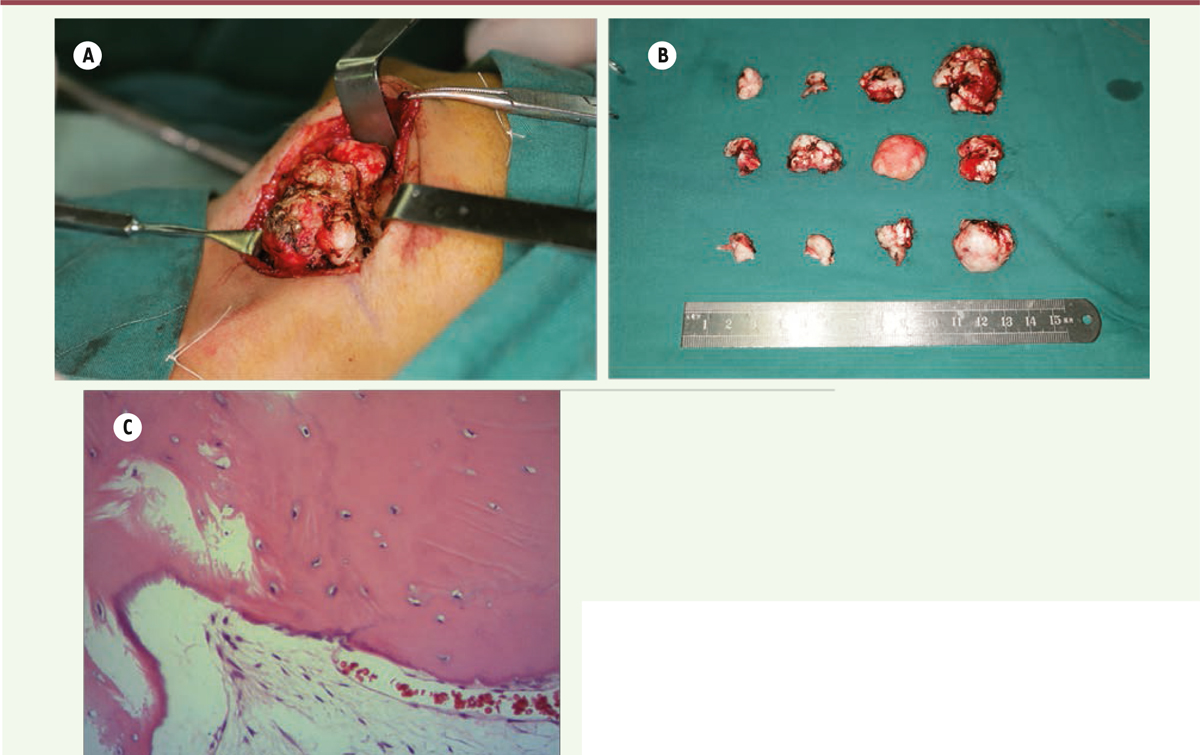
The proposed treatment plan was excision of the major osteomas via an external approach under general anesthesia. The major osteomas located at bilateral mandibular angles and rami were removed via submandibular approach. This access allowed sufficient exposure of most osteomas on the mandibular ramus and angle (Figure 3A). The resection of these osteomas was difficult due to its very hard cortical nature (Figure 3B). The smaller osteomas were not removed as they were insignificant to the objective of improving the mandibular contour of the patient. The wounds were closed and suction drains were left for 3 days. The resected specimens were sent for histopathological analysis. Histopathological results revealed that the osteomas contained osseous nodules with irregular Haversian canals without any osteoclasts or osteoblasts (Figure 3C). The surrounding tissues showed a large number of small blood vessels. Simultaneous removal of the soft tissue lumps in his scalp was performed in the same surgery. The pathological diagnosis was epidermoid cyst with pilomatricoma-like changes (Figure 1B). There were no surgical complications. Postoperative CT scan showed the contour of mandible was regular and symmetrical (Figure 2C). The patient was satisfied with the esthetic improvement after the surgery.
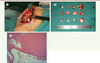 | Figure 3. (A) Exposed mass on mandibular angle region showed the clustered osteomas. (B) Macroscopic view of the resected masses, the largest one was about 30mm in diameter. (C) Histopathological results revealed that the osteomas are constituted of osseous nodule (hematoxylin-eosin stain, 20x). |
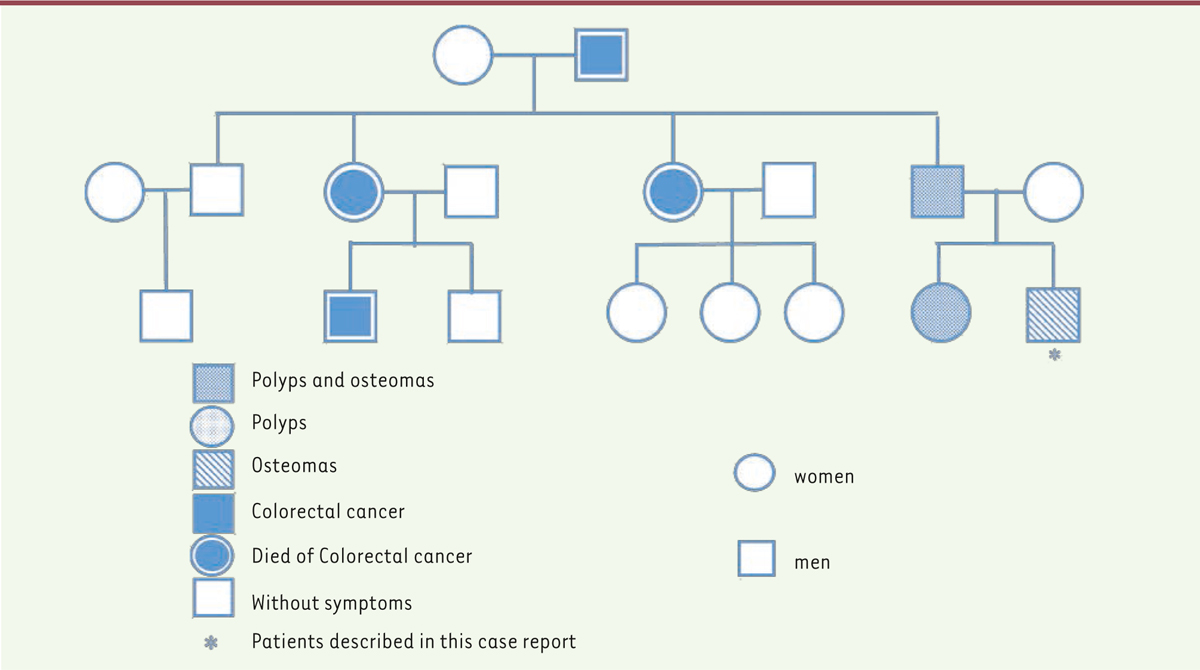
Based on the family history, clinical, radiographic and histopathological findings, a working diagnosis of GS was reached. Subsequently, colonoscopy was performed and showed normal intestinal mucosa (Figure 1C). A genetic screening was performed which showed a mutation in codons 15 to 17 at exon 15 of the APC gene. Upon confirmation of the diagnosis of GS, to slow down the development of the adenomatous polyps, Arthrocine 200mg/day was prescribed. The patient was recommended to undergo annual radiographic examination of the jaws and annual colonoscopy to closely monitor for intestinal polyposis. No signs of relapse in the jaws and no intestinal polyp were observed on one-year follow-up. A family pedigree of this syndrome was plotted (Figure 4). In this family, four members across three generations have died from intestinal cancer. His father had undergone a colectomy for adenomatous polyposis and his sister received no treatment. We proposed gene screen, colonoscopy, as well as radiographic examination of the craniomaxillofacial skeleton for both the sister and father. This was declined by the father due to financial reasons and by the sister as she was pregnant.
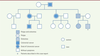 | Figure 4. The family tree showing affected members. |
|
GS results from the mutation on the APC gene, which is located on the long arm of chromosome 5 (5q21-22). The APC gene was identified in 1991 and modifier genes responsible for the development of the extraintestinal manifestations are currently still enigmatic. In approximately 75% of the cases, a defective gene is inherited from one of the parents. In the remaining 25%, the mutations are sporadic [1]. Over 300 different mutations have been reported [3]. Using molecular genetic techniques, doctors can screen for the pre-symptomatic members of a family carrying the mutated APC gene. As a guideline, the offspring of individuals suffering from the syndrome should undergo a comprehensive examination on their tenth and fifteenth birthdays [1]. Usually appearing between 13 and 31 years of age, adenomatous polyps can occur anywhere in the gastrointestinal tract except the esophagus, normally in the colon and the rectum. Gastric polyps are rare. These polyps can cause diarrhea, constipation, intestinal pain, or rectal bleeding. The adenomatous polyps are associated with colossal risk (almost 100%) of malignant degeneration if they are not resected [1]. Malignant changes occur within 10 to 15 years after the onset of the lesions [7]. On the average, degeneration of these polyps occurs around 37 years of age [8]. Approximately 25% of patients with FAP have colorectal cancer at diagnosis [8]. It is believed that most patients with intestinal polyposis die by age 50, unless they have undergone surgical treatment [6]. Consequently, early diagnosis and treatment is critical. Some experts recommend the use of medication to reduce the number and size of these polyps, such as tamoxifen and non-steroidal anti-inflammatory drugs e.g. Sulindac [1]. Annual colonoscopy is advised until age 35 and every 2-3 years thereafter [1]. Katou F et al [9] estimated that over 70% of patients suffering from GS have osteomas. Osteomas precede clinical and radiological evidence of colonic polyposis; therefore, they may be sensitive markers for the disease [9]. These benign neoplasms comprise of well-differentiated compact or cancellous bone characterized by slow, continuous growth. They can be sub-classified into central osteomas arising from the endosteum (enostoses) and peripheral osteomas (exostoses) arising from the periosteum [1]. The radiographic features of exostoses are characterized by round or oval radiopaque masses attached by a broad base, whereas enostoses appear as large and diffuse radiopaque cotton wool-like lesions in the jaw [10]. CT is a helpful tool in estimating the size of the lesion and establishing the relation between the tumor and any important adjacent structures [1]. Bone scintigraphy can reveal the presence of an active bony lesion, showing the presence of multiple foci of increased uptake in the craniofacial bones. While a solitary osteoma of the jaw is a common incidental finding on dental panoramic tomography, if more than three such lesions are found, it is highly suggestive of GS [11]. Osteomas are benign tumors which may cause disfigurement and dysfunction and are associated with a low rate of malignant transformation and post-resection recurrence rate. Oliveira et al [12] reported a case of limited mouth opening due to bilateral coronoid hyperplasia that was later diagnosed to be GS. Surgical treatment of osteomas with dysfunction is necessary. Jaw lesions grow gradually in childhood and adolescence and continue to develop in adulthood. Periodical radiographic examination of the jaws is recommended during postoperative follow-up [13]. As our patient’s concerns were primarily esthetic, only osteomas on bilateral rami and angles were resected. The patient was satisfied with the result. GS is associated with dental anomalies such as supernumerary teeth, congenitally missing teeth, impacted teeth and odontoma which can be picked up by routine radiographs. Dental anomalies are present in 30-75% of GS patients. In some cases, the preliminary diagnosis of GS is made by dentists. The most common ectodermal abnormalities of GS are epidermoid cysts, frequently associated with lipoma, desmoid tumors, fibroma, leiomyomas, neuro-fibroma, or pigmented skin [1]. Epidermoid cysts are present in approximately 50-65% of patients, arising prior to puberty and occur primarily on the face, scalp and extremities, with no malignant potential [14]. Although the diagnosis of GS can only be confirmed by gastrointestinal endoscopy and molecular genetic analysis, it may be guided by immune-histochemical profile of extra-intestinal lesions. A fibroma with tumor cells stained by anti-CD34 and beta-catenin antibodies would suggest a Gardner fibroma [15]. In this reported case study, four family members died of intestinal cancer without the diagnosis of GS. The diagnosis of GS was never known until the patient was referred under our care. He presented with multiple osteomas and dental anomalies in the craniofacial region along with epidermoid cysts, with no intestinal polyps currently. A literature search returned 18 well-documented cases of GS which reported clearly the first clinical feature(s), which may include osteomas, epidermal cysts, FAP, desmoid tumors, dental anomalies and/or adenocarcinoma of the rectum (Table 1). Osteomas and dental anomalies present as the first clinical signs of GS in 53% of these cases. To avoid missing the diagnosis of GS, it is important for otolaryngologists, dentists and oral surgeons to be familiar with the common manifestations of GS.
Table 1
| Case Study |
Age/Gender |
First clinical feature(s) |
Country |
| #1 Oliveira et al [12] |
12/Male |
Osteomas |
Brazil |
|
| #2 Xuereb S et al [16] |
18/Male |
Epidermal cysts |
Maltese |
|
| #3 Álvarez Salgado JA et al [17] |
19/Male |
Osteoma |
Brazil |
|
| #4 Verma P et al [18] |
52/Male |
Osteomas |
India |
|
| #5 Ragupathy K et al [19] |
45/Male |
Osteomas |
India |
|
| #6 Schmoyer CJ et al [20] |
20/Female |
Aggressive fibromatosis |
USA |
|
| #7 Ikai A et al [21] |
22/Female |
FAP |
Japan |
|
| #8 D M et al [22] |
29/Male |
Mesenteric Fibromatosis (Desmoid Tumor) |
not mentioned |
|
| #9 Singh K et al [23] |
25/Male |
Dental anomalies |
not mentioned |
|
| #10 Punatar SB et al [24] |
40/Male |
adenocarcinoma of the rectum |
not mentioned |
|
| #11 Ali SY et al [25] |
18/Female |
Multiple skin lesions |
India |
|
| #12 Rekik NM et al [26] |
30/Female |
FAP |
not mentioned |
|
| #13 Lanckohr C et al [27] |
13/Male |
Gardner fibroma and osteoma |
not mentioned |
|
| #14 Cabassa P et al [28] |
38/Male |
Adenocarcinoma of the rectum |
not mentioned |
|
| #15 Gu GL et al [29] |
23/Female |
FAP |
China |
|
| #16 Smud D et al [30] |
15/Female |
Osteomas |
not mentioned |
|
| #17 de Oliveira Ribas M et al [31] |
25/Male |
Osteomas |
Brazil |
|
| #18 de Oliveira Ribas M et al [31] |
12/Female |
Osteomas and Dental anomalies |
Brazil |
Summary of the 18 case reports of Gardner’s syndrome.
|
|
The authors declare no conflict of interests with respect to the present article. |
1. Ben Lagha Nadia, Galeazzi Jean-Marc, Chapireau David, Oxeda Pascal, Bouhnik Yoram, Maman Louis. Surgical management of osteoma associated with a familial Gardner’s syndrome . J Oral Maxillofac Surg. 2007; ; 65 : (6) :1234.–1240. 2. Wijn MA, Keller JJ, Giardiello FM, Brand HS. Oral and maxillofacial manifestations of familial adenomatous polyposis . Oral Dis. 2007; ; 13 : (4) :360.–365. 3. Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate . Hum Mutat. 1994; ; 3 : (2) :121.–125. 4. Cristofaro Maria Giulia, Giudice Amerigo, Amantea Massimiliano, Riccelli Umberto, Giudice Mario. Gardner’s syndrome: a clinical and genetic study of a family. Oral Surg Oral Med Oral Pathol . Oral Radiol. 2013; ; 115 : (3) :e1.–e6. 5. BülowS. Familial polyposis coli . Dan Med Bull. 1987; ; 34 : (1) :1.–15. 6. Ida M, Nakamura T, Utsunomiya J. Osteomatous changes and tooth abnormalities found in the jaw of patients with adenomatosis coli . Oral Surg Oral Med Oral Pathol. 1981; ; 52 : (1) :2.–11. 7. Carl W, Herrera L. Dental and bone abnormalities in patients with familial polyposis coli . Semin Surg Oncol. 1987; ; 3 : (2) :77.–83. 8. Abraham Susan C, Park Seun Ja, Mugartegui Lilian, Hamilton Stanley R, Wu Tsung-Teh. Sporadic fundic gland polyps with epithelial dysplasia: evidence for preferential targeting for mutations in the adenomatous polyposis coli gene . Am J Pathol. 2002;;161((5))::1735.–1742. 9. Katou F, Motegi K, Baba S. Mandibular lesions in patients with adenomatosis coli . J Craniomaxillofac Surg. 1989; ; 17 : (8) :354.–358. 10. Kubo K, Miyatani H, Takenoshita Y, Abe K, Oka M, Iida M. Widespread radiopacity of jaw bones in familial adenomatosis coli . J Craniomaxillofac Surg. 1989; ; 17 : (8) :350.–353. 11. Payne M, Anderson JA, Cook J. Gardner’s syndrome - a case report . Br Dent J. 2002; ; 193 : (7) :383.–384. 12. Oliveira MR, Rodrigues WC, Gabrielli MF, Gabrielli MA, Onofre MA, Filho VA. Gardner Syndrome With Unusual Maxillofacial Manifestation . J Craniofac Surg. 2016; ; 27 : (5) :1253.–1255. 13. Timuçin Baykul, Nurettin Heybeli, Orhan Oyar. Dog˘ru Harun. Multiple huge osteomas of the mandible causing disfigurement related with Gardner’s syndrome: case report . Auris Nasus Larynx. 2003; ; 30 : (4) :447.–451. 14. Perniciaro C.. Gardner’s syndrome . Dermatol Clin. 1995; ; 13 : (1) :51.–56. 15. Guignard N, Cartier C, Crampette L, Akkari M. Gardner’s syndrome presenting with a fibromatous tumour of the parotid . Eur Ann Otorhinolaryngol Head Neck Dis. 2016; ; 133 : (5) :357.–359. 16. Xuereb S, Xuereb R, Buhagiar C, Gauci J, Magri C. A case report of desmoid tumour-a forgotten aspect of FAP? . Int J Surg Case Rep. 2017; ; 30 : :122.–125. 17. Álvarez Salgado JA, Villaseñor Ledezma, JJ., Cañizares Méndez, M. L., Paredes, S. I., Rodríguez, d. L. A., Mollejo, V. M. Ectopic craniopharyngioma and Gardner’s syndrome: Case report and literature review . Neurocirugia (Astur). 2017;;28((2))::97.–101. 18. Verma P, Surya V, Kadam S, Umarji HR. Classical presentation of Gardner’s syndrome in an Indian patient: A case report . Contemp Clin Dent. 2016; ; 7 : (2) :277.–280. 19. Ragupathy K, Priyadharsini I, Sanjay P, Yuvaraj V, Balaji TS. Peripheral Osteoma of the Body of Mandible: A Case Report . J Maxillofac Oral Surg. 2015; ; 14 : (4) :1004.–1008. 20. Schmoyer CJ, Brereton HD, Blomain EW. Contralateral recurrence of aggressive fibromatosis in a young woman: A case report and review of the literature . Oncol Lett. 2015; ; 10 : (1) :325.–328. 21. Ikai A, Fujiwara H, Shiozaki A, Okamoto K, Kosuga T, Konishi H, Komatsu S, Ichikawa D, Morimura R, Kuriu Y, Ikoma H, Nakanishi M, Ochiai T, Sakakura C, Otsuji E. Gastric cancer arising from gastric polyps in gardner syndrome - a case report . Gan to Kagaku Ryoho. 2014; ; 41 : (12) :2262.–2263. 22. Ghalige HS, Sharma MB, Singh TS. Mesenteric Fibromatosis (Desmoid Tumour) - A Rare Case Report . J Clin Diagn Res. 2014;;8((11))::ND01.–2. 23. Singh K, Singh A, Kumar P, Gupta N. Prosthodontic management of a patient with Gardner’s syndrome: A clinical case report . Dent Res J (Isfahan). 2014; ; 11 : (2) :276.–280. 24. Punatar SB, Noronha V, Joshi A, Prabhash K. Thyroid cancer in Gardner’s syndrome: Case report and review of literature . South Asian J Cancer. 2012; ; 1 : (1) :43.–47. 25. Ali SY, Prabhat S, Ramanamurty ChV, Salma M, Hussain S, Murtaza AS. Coexistence of porokeratosis of Mibelli with Gardner’s syndrome: A rare case report . Indian Dermatol Online J. 2011; ; 2 : (2) :94.–96. 26. Rekik NM, Ben Salah S, Kallel N, Kamoun M, Charfi N, Abid M. Adrenocortical Secreting Mass in a Patient with Gardner’s Syndrome: A Case Report . Case Rep Med. 2010;;2010::682081.. 27. Lanckohr C, Debiec-Rychter M, Müller O, Homann HH, Lehnhardt M, Herter P, Kuhnen C. Gardner fibroma: case report and discussion of a new soft tissue tumor entity . Pathologe. 2010; ; 31 : (2) :97.–105. 28. Cabassa P, Morone M, Gatti E, Narbone M, Maroldi R. Gardner syndrome complicated with hydronephrosis. A case report . J Radiol Case Rep. 2010;;4((3))::19.–23. 29. Gu GL, Wang SL, Wei XM, Bai L. Diagnosis and treatment of Gardner syndrome with gastric polyposis: a case report and review of the literature . World J Gastroenterol. 2008; ; 14 : (13) :2121.–2123. 30. Smud D, Augustin G, Kekez T, Kinda E, Majerovic M, Jelincic Z. Gardner’s syndrome: genetic testing and colonoscopy are indicated in adolescents and young adults with cranial osteomas: a case report . World J Gastroenterol. 2007; ; 13 : (28) :3900.–3903. 31. de Oliveira Ribas M, Martins WD, de Sousa MH, de Aguiar Koubik AC, Avila LF, Zanferrari FL, Martins G. Oral and maxillofacial manifestations of familial adenomatous polyposis (Gardner’s syndrome): a report of two cases . J Contemp Dent Pract. 2009; ; 10 : (1) :82.–90. |