| |
| Med Sci (Paris). 34: 33–38. doi: 10.1051/medsci/201834f106.Lycorine inhibits melanoma A375 cell growth and metastasis through the inactivation of the PI3K/AKT signaling pathway Qun-Qun Jiang1* and Wei-Bing Liu1 1Department of Dermatology, 404 Hospital of People’s Liberation Army, No.8 of Baoquan Street, Huancui District, Weihai264200, Shandong Province, China |
Malignant melanoma, one of the most aggressive skin cancers, has a very high mortality rate. Currently, the number of drugs to treat melanoma is low. Although new immunotherapeutic approaches based on the use of antibodies against immune checkpoints have shown long term responses, it is urgent to develop novel anti-melanoma drugs with a high efficiency and a low toxicity in a large number of patients. Lycorine, a natural product, has been reported to exert antitumor effects on some cancers. However, the impact of lycorine on melanoma cells is still unknown. Using the CCK8 assay, we found that lycorine can suppress the proliferation of melanoma A375 cells in a dose-time-dependent manner. Moreover, a transwell assay showed that lycorine inhibited the migration and invasion of A375 cells significantly. Further, lycorine treatment could induce the apoptosis of the A375 cells. Biochemical analyses showed that the expression level of the anti-apoptosis Bcl-2 protein decreased, while the expression of the pro-apoptosis protein Bax and active caspase-3 increased after lycorine treatment. Finally, using western blot assay, we found that the antitumor effects of lycorine on A375 cells might be through the inactivation of the PI3K/Akt signaling pathway. Based on these observations, we suggest that lycorine may be an interesting candidate for further studies on its ability to represent a novel antitumor drug for human melanoma treatment in the future. Keywords: Lycorine, melanoma, proliferation, migration, apoptosis, PI3K/AKT signaling pathway |
Malignant melanoma is aggressive, and its incidence has been rising worldwide over the past three decades [1, 2]. Although a lot of efforts have been made to improve melanoma treatment recently, the overall median survival of melanoma patients is only 4-6 months, even though the use of anti-immune checkpoint antibodies has shown remarkable results in about 20% of the patients, with long-term survival. Tumor metastasis and the lack of effective drugs are the main reason for the poor overall survival rate [2-4]. Thus, there is an urgent need to find some novel effective drugs to inhibit the proliferation and metastasis of melanoma cells. Natural products possess multiple bioactivities and serve as excellent drug leads for cancer treatment [5-7]. Lycorine, an active alkaloid extracted from a common herbal medicine (Lycoris radiate), was reported to possess anti-viral, anti-inflammatory and anti-cancer activities [8-10]. Notably, it has been found that lycorine can suppress the development of various malignant tumors, including multiple myeloma, hepatocellular carcinoma, and prostate cancer [11-13]. However, whether lycorine can target melanoma cells remains still unclear. In this study, using melanoma A375 cells as target cells, we found that lycorine has an antitumor effect on melanoma growth and invasion property in vitro. This could be through the blockade of the PI3K/AKT signaling pathway and the induction of apoptosis as also reported in this issue by Li and Huang when the JTC-801 drug was evaluated in vitro using the M14 melanoma cells. |
Chemicals and reagents Lycorine (purity > 98%) was purchased from Shanghai Yuanye Bio-Technology (Shanghai, China). A 40 mM stock solution was prepared in dimethyl sulfoxide (DMSO) and stored at −20°C in aliquots. Dimethyl sulfoxide (DMSO) was purchased from Sigma (St. Louis, MO, USA). RPMI-1640 medium, fetal calf serum (FCS), L-glutamine were purchased from GIBCO Invitrogen Life Technologies (Carlsbad, USA). Primary antibodies against AKT, p-AKT, mTOR, p-mTOR, 4EBP1, Bax, Bcl-2, Active caspase-3 and actin were purchased from Cell Signaling Technology, Inc. (Beverly, USA). The enhanced chemiluminescence (ECL) detection system was obtained from Millipore. (Millipore, USA). Cell culture The HEMa and A375 human melanoma cancer cell lines were purchased from ATCC. A375 cells were maintained at 37°C, in a 5% CO 2 humidified incubator, in RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 100 U/mL penicillin and 0.1 mg/mL streptomycin. HEMa cells were cultured in serum-free M254 medium supplemented with human melanocyte growth supplement (HMGS). Western blot Cells were homogenized in ice-cold RIPA buffer, which contained 25 mM Tris-HCl (pH 7.6), 0.1% sodium dodecyl sulfate (SDS), 150 mM NaCl, 1% NP-40 and 1% sodium deoxycholate with phosphatase and protease inhibitors. Lysates were centrifuged at 14,000 × g for 15 min at 4 °C. The amounts of protein from each treated cells were detected and normalized using the BCA assay. Equal amounts of protein (20 μg/lane) were loaded onto SDS-PAGE gels and subsequently transferred to PVDF membranes. The blots were then incubated separately overnight at 4 °C with primary antibodies directed against AKT, p-AKT, mTOR, p-mTOR, 4EBP1, Bcl-2, Bax, Active-caspase3 and actin. After washing with TBST buffer, the membranes were incubated with a secondary antibody (Proteintech) for 2h at room temperature. The blots were then revealed using the ECL detection system (Millipore). Densitometric analyses of the bands were performed using the Quantity One software (Version 4.5.0.). The proteins were quantified and the results expressed as a ratio to GAPDH quantity. Experiments were repeated five times. Cell Counting Kit 8 (CCK-8) assay Normal human primary epidermal melanocytes (HEMa) and A375 cells were seeded in 96-well plates at a density of 1,000 cells/well in 100μL of culture medium with different lycorine (0, 2, 10, 50 μM) doses for 72 h. Twenty-four, 48 h, 72 h and 96 h after lycorine (at 50 μM) treatment, 10 μL CCK-8 solution were added to each well and incubated for 1.5 hours at 37 °C. The optical density (O.D.) was measured at 450 nm using a microplate reader. The experiment was repeated 3 times. Transwell migration and invasion assay Migration and invasion assays were carried out using 24-well transwell chambers plate (Millipore, USA). For invasion experiment, Matrigel (BD, USA) was dissolved overnight in serum-free RPMI-1640 medium (diluted 1:6). 100 μl were then added into the upper part of the chamber. After shaking evenly, the plate was incubated into a CO 2 incubator for 4-6 h at 37 °C until gel formation. 1 × 10 5 cells per well in 100 μL of serum-free medium were pretreated with lycorine and loaded into the upper chamber. 500 µL medium with 10% FBS were then added to the lower chamber. After incubation for 48 h at 37 °C into a CO 2 incubator, cells remaining in the upper chamber were removed with a cotton swab. Cells that had crossed the membrane and present in the lower chambers were fixed and stained in 4% paraformaldehyde and 0.1% crystal violet solution. Five random fields (x 200) were examined under the microscope. The number of invasive or migratory cells was then determined and the mean values were calculated. Each experiment was performed three times. The migration experimental procedure was similar to the invasion assay, with no matrigel in the chambers. Flow cytometric analysis After being treated with lycorine for 24 h, cells were harvested, washed twice with PBS, and were then resuspended in Annexin V binding buffer and incubated with Annexin V-FITC/PI (Beijing 4A Biotech, China) in the dark for 15 min at room temperature. The percentages of apoptotic cells were determined using flow cytometry. Results were analyzed by Flowjo software. Experiments were repeated five times. Statistical analysis Statistical analyses were performed using the SPSS 18.0 software. All data were presented as the mean ± standard deviation (S.D.). Data of only two groups were analyzed with the Student’s t-test. One-way ANOVA followed by the LSD test was used for three or more groups analysis. P values < 0.05 were considered as statistically significant. |
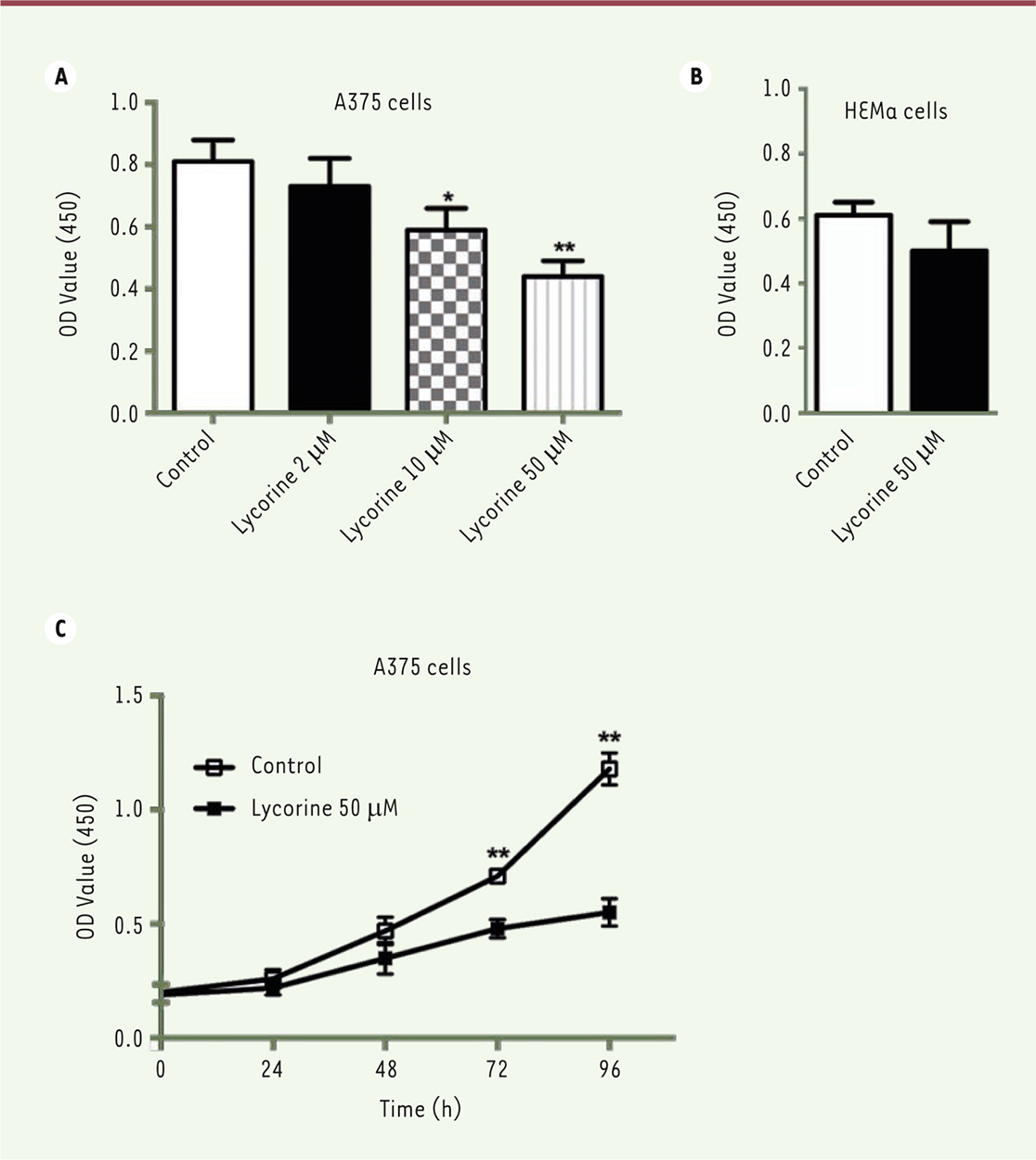
Lycorine inhibits the proliferation of the A375 melanoma cells Firstly, a CCK8 assay was used to examine the effect of lycorine on the proliferation of A375 cells. After being incubated with different doses of lycorine (2, 10, 50 μM) for 72 h, the viability of A375 cells decreased significantly in a dose-dependent manner (p<0.01). The inhibition was the strongest with 50 μM lycorine (p<0.01). Moreover, only a marginal cytotoxicity was observed when normal melanocytes HEMa were cultured with 50 μM lycorine. Thus, 50 μM lycorine were used in the following experiments. Next, the effect of lycorine on the proliferation of A375 cells at different time points was examined. The O.D. values after 48h and 72h licorine treatment were significant lower compare to those observed with the NC group (both p<0.05). It suggests that the proliferation of A375 is also inhibited by lycorine in a time-dependent manner (Figure 1).
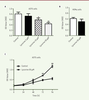 | Figure 1. Lycorine inhibits the proliferation of A375 cells in a dose- and time-dependent manner (CCK-8 assay). A. Effects of different doses of lycorine on A375 cell proiferation. B. Effect of 50 μM lycorine on HEMa cell (normal melanocytes) proliferation. C, Effects of 50 μM lycorine on A375 cell proliferation after lycorine treatment for different periods of time. All values are presented as means ± S.D. *P<0.05, ** P<0.01 compared with control group. n = 3 for each group. |
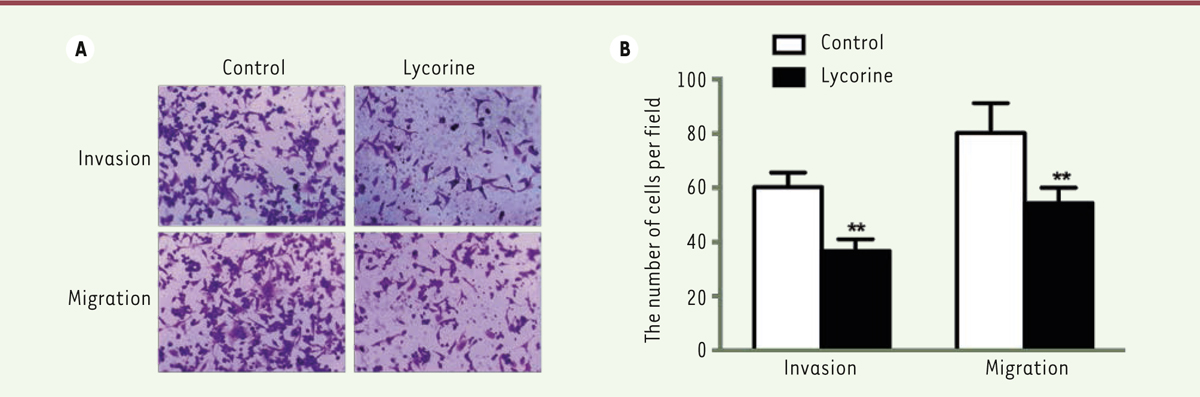
Lycorine suppresses the migration and invasion of A375 cells Then we evaluated the effect of lycorine on migration and invasion of A375 cells by the transwell migration and invasion assays (Figure 2). The numbers of migrated and invasive cells in the lycorine-treated cell group were lower than those of the NC group (both p< 0.01). These results suggest that lycorine efficiently inhibits the migration and invasion of the A375 melanoma cells.
 | Figure 2. Lycorine inhibits A375 cell invasion and migration. A. A375 cell invasion and migration after lycorine treatment (x200 magnification). B. Statistical graph for A375 cell invasion and migration after lycorine treatment. ** P<0.01 compared with control group. All values are presented as means ± S.D. n = 3 for each group. |
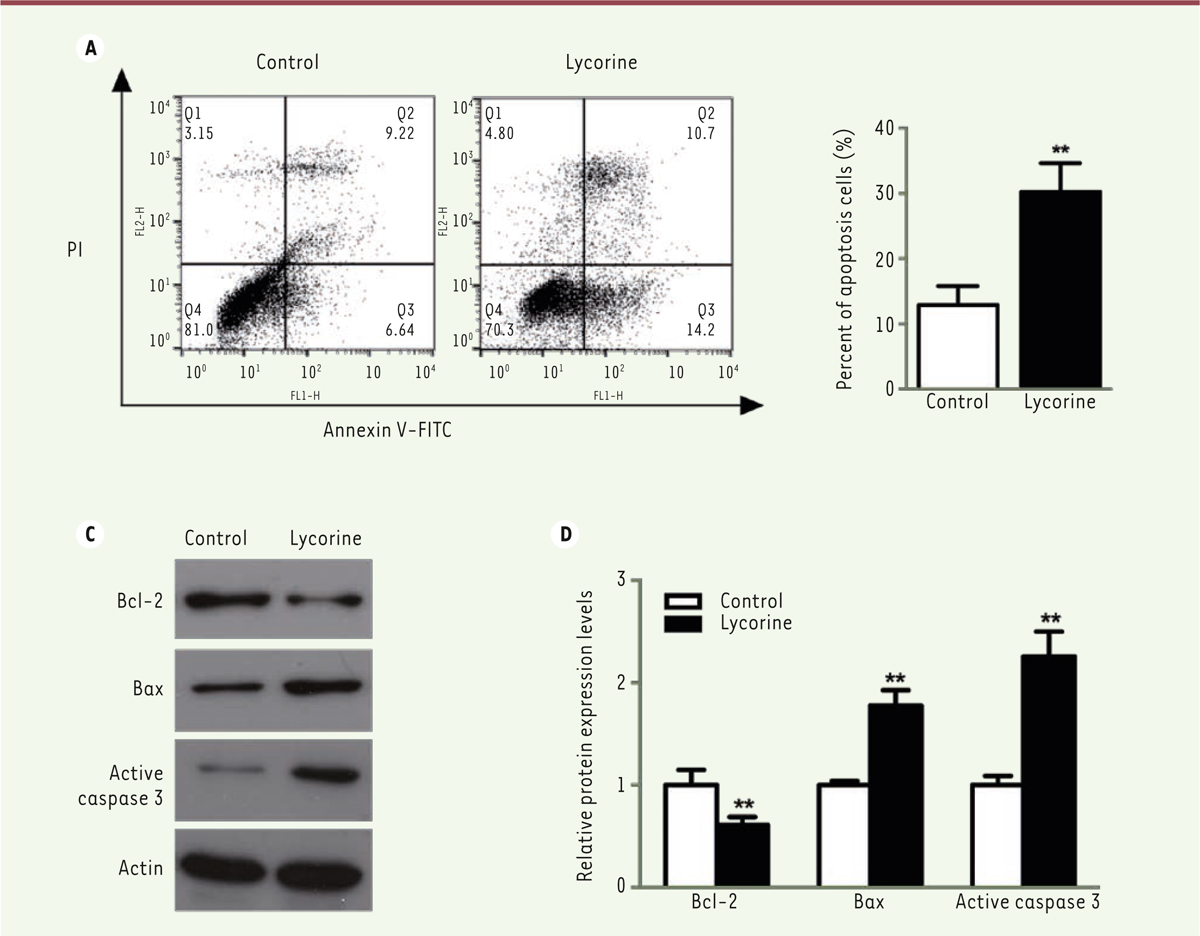
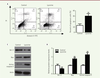 | Figure 3. Lycorine induces A375 cell apoptosis. A. A375 cells analysis by flow cytometry after double Annexin V/FITC staining. B. quantitative analyses of the flow cytometry results shown in A (A375 cell apoptosis). C. Western blot of apoptosis-related proteins in lycorine-treated A375 cells. D. quantitative analysis of the Western blot images shown in C. The relative protein expression levels were normalized to the actin protein levels. ** P<0.01 compared with control group. All values are presented as means ± S.D. n = 5 for each group. |
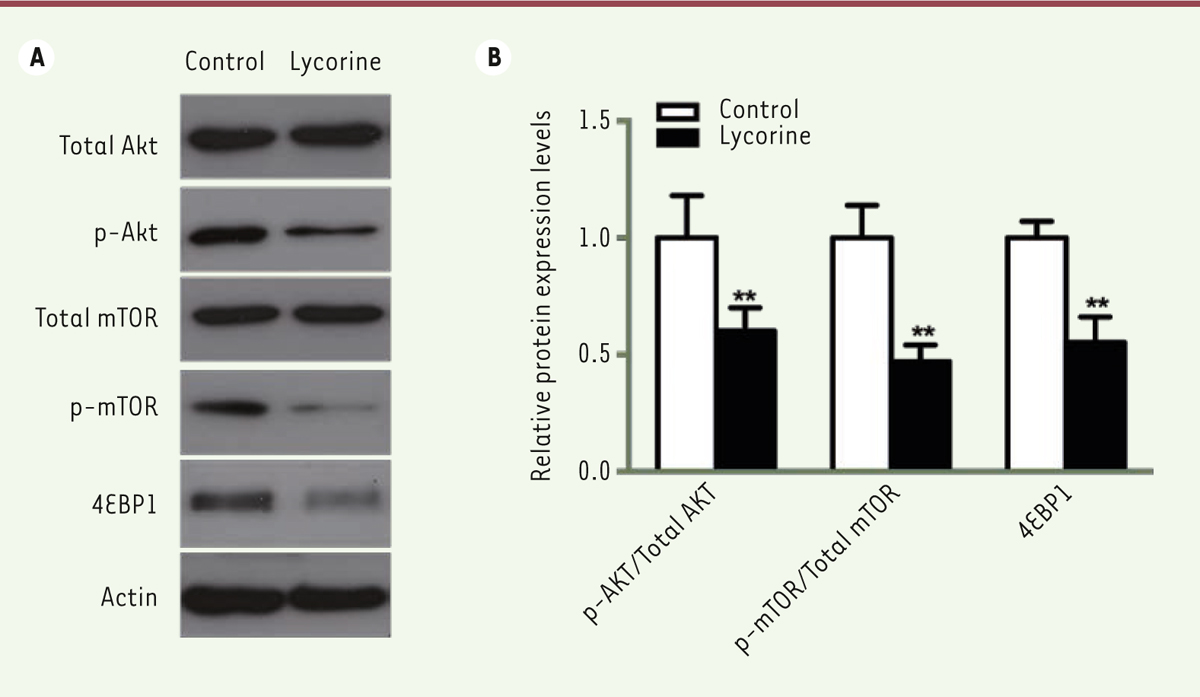
Lycorine promotes the apoptosis of A375 cells We used a double Annexin V-FITC / PI staining and a western blot assay to verify whether the effect of lycorine on A375 cells was correlated with cell apoptosis. Flow cytometry analysis showed that the percentage of apoptotic cells after lycorine treatment was significantly increased (p< 0.05). We then analyzed the changes in the expression levels of apoptosis-related Bcl-2, Bax and Active caspase-3 molecules after lycorine treatment by western blotting. The expression level of the anti-apoptotic protein Bcl-2 was decreased. However, the expression levels of the pro-apoptotic protein Bax and of Active Caspase-3 were increased (p< 0.05). All these results indicated that lycorine induces the apoptosis of A375 cells at least in vitro. Lycorine inhibits the PI3K/Akt signaling pathway in A375 cells PI3K/Akt /mTOR signaling pathway is one of the key pathway related to cell proliferation, survival and metabolism. Abnormal expression and dysregulation of this pathway are frequently observed in various types of tumors. To analyze whether the effect of lycorine on cells was through the PI3K/Akt/mTOR signaling pathway, we examined the expression changes of key proteins of this pathway by western blot (Figure 4). We found that the phosphorylation of Akt and mTOR was significantly inhibited in A375 cells treated with lycorine (p<0.05). In addition, lycorine treatment down-modulated the activity of 4EBP1, a downstream protein in the PI3K signaling pathway, by decreasing its expression (p<0.05). Thus, lycorine treatment inhibits the PI3K/AKT/mTOR signaling pathway in A375 cells.
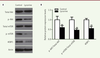 | Figure 4. Lycorine inactivates the PI3K/AKT/mTOR signaling pathway in A375 cells. A. Western blot of PI3K/AKT/mTOR signaling pathway-related proteins in lycorine-treated A375 cells. B. quantitative analysis of the Western blot images shown in A. ** P<0.01 compared with control group. All values are presented as means ± S.D. n = 5 for each group. |
|
In the present study, we show that lycorine suppresses the proliferation, migration and invasion of melanoma A375 cells effectively. Moreover, the apoptosis of A375 cells is increased after lycorine treatment. These effects of lycorine in A375 cells might be regulated through the inhibition of the PI3K signaling pathway. As a natural alkaloid, lycorine exists in the flowers and bulbs of some Amaryllidaceae species, including spider lilies (Lycoris), daffodils (Narcissus) and snowdrops (Galanthus) [14]. Although the potential targets or underlying mechanisms of lycorine action are still unclear, lycorine is a promising potential clinical drug or lead for tumor treatment because of its various biological effects and low cytotoxicity [15, 16]. Thus, the role of lycorine in melanoma needed to be further clarified. In our study, we found that lycorine suppresses proliferation, migration and invasion of A375 cells effectively, which are consistent with findings reported in breast cancer, bladder cancer and myeloma [11, 17, 18]. All these results suggest that lycorine can be a promising candidate for the treatment of skin cancer in the future. Tumor cells grow rapidly due to the activation of the cell cycle progression. Cell cycle arrest is one of the mechanisms that has been advocated to inhibit tumor growth [19, 20]. Some studies show that the antitumor effects of lycorine might be related with an accelerated apoptosis [13, 18]. Consistant with these reports, using flow cytometry analysis, we found that lycorine significantly increases the percentage of apoptotic A375 cells. In addition, the expression of the anti-apoptotic protein Bcl-2 decreased, while the expression of the pro-apoptotic protein Bax and of Active Caspase-3 increased. These results indicate that lycorine could induce the apoptosis of melanoma cells. The lack of toxicity observed when the HEMa normal melanocytes were tested suggests that licorine could play an efficient role against melanoma tumor cells. Many reports have shown that the PI3K/Akt signaling pathway is involved in multiple cellular functions, including cell proliferation, apoptosis and invasion [21-23]. Thus, the PI3K/Akt pathway is usually regarded as one of the key target for various tumors treatment [24-26], including melanoma. Calero et al indicated that the combination of the HSP90 inhibitor 17AAG with the PI3K/mTOR inhibitor NVP-BEZ235 has a synergistic activity in decreasing melanoma cell growth, inducing apoptosis and targeting simultaneously the MAPK and PI3K/Akt/mTOR pathways [27]. In addition, a study suggests that baicalein, a natural flavonoid, inhibits B16F10 melanoma cell migration and invasion, possibly by inhibiting the phosphoinositide 3-kinase/Akt signaling pathway [28]. However, whether lycorine inhibits A375 cells proliferation, apoptosis and invasion through a down-modulation of the PI3K/Akt signaling pathway was still unknown. In the present study, we found that the phosphorylation of Akt and mTOR was significantly inhibited in A375 cells treated with lycorine. The amount of p70S6K and Cyclin D1, two downstream targets of Akt, was also inhibited in lycorine-treated A375 cells. These data strongly suggest that the anti-melanoma effect of lycorine might take place through the down-modulation of the PI3K/Akt/mTOR signaling pathway. In conclusion, lycorine induces the apoptosis of A375 cells and inhibit its proliferation, invasion and migration in vitro, likely through the inactivation of the PI3K signaling pathway. Therefore, our data indicate that lycorine might be a drug candidate for the treatment of melanoma. More works are needed to confirm in vitro with other melanoma cell lines and in vivo using preclinical animal models the anti-melanoma activity of licorine. |
The authors declare that they have no competing interests. |
1. Pandiani C, Beranger GE, Leclerc J, et al. Focus on cutaneous and uveal melanoma specificities . Genes Dev. 2017; ; 31 : :724.–743. 2. Millet A, Martin AR, Ronco C, et al. Metastatic Melanoma: Insights Into the Evolution of the Treatments and Future Challenges . Med Res Rev. 2017; ; 37 : :98.–148. 3. Lim SY, Menzies AM, Rizos H. Mechanisms and strategies to overcome resistance to molecularly targeted therapy for melanoma . Cancer. 2017; ; 123 : :2118.–2129. 4. Volpe VO, Klufas DM, Hegde U, Grant-Kels JM. The new paradigm of systemic therapies for metastatic melanoma . J Am Acad Dermatol. 2017; ; 77 : :356.–368. 5. Shanmugam MK, Lee JH, Chai EZ, et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds . Semin Cancer Biol. 2016; ; 40–41 : :35.–47. 6. Xiao Z, Morris-Natschke SL, Lee KH. Strategies for the Optimization of Natural Leads to Anticancer Drugs or Drug Candidates . Med Res Rev. 2016; ; 36 : :32.–91. 7. Fangjun L, Zhijia Y. Tumor suppressive roles of eugenol in human lung cancer cells . Thorac Cancer. 2017. 8. Ghavre M, Froese J, Pour M, Hudlicky T. Synthesis of Amaryllidaceae Constituents and Unnatural Derivatives . Angew Chem Int Ed Engl. 2016; ; 55 : :5642.–5691. 9. Kang J, Zhang Y, Cao X, et al. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge . Int Immunopharmacol. 2012; ; 12 : :249.–256. 10. Guo Y, Wang Y, Cao L, et al. A Conserved Inhibitory Mechanism of a Lycorine Derivative against Enterovirus and Hepatitis C Virus . Antimicrob Agents Chemother. 2016; ; 60 : :913.–924. 11. Roy M, Liang L, Xiao X, et al. Lycorine Downregulates HMGB1 to Inhibit Autophagy and Enhances Bortezomib Activity in Multiple Myeloma . Theranostics. 2016; ; 6 : :2209.–2224. 12. Hu M, Peng S, He Y, et al. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer . Oncotarget. 2015; ; 6 : :15348.–15361. 13. Yu H, Qiu Y, Pang X, et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma . Mol Cancer Ther. 2017. 14. Subramaniam S, Sundarasekar J, Sahgal G, Murugaiyah V. Comparative analysis of lycorine in wild plant and callus culture samples of Hymenocallis littoralis by HPLC-UV method . ScientificWorldJournal. 2014; ; 2014 : :408306.. 15. Nair JJ, van Staden J, Bastida J. Apoptosis-Inducing Effects of Amaryllidaceae Alkaloids . Curr Med Chem. 2016; ; 23 : :161.–185. 16. Nair JJ, van Staden J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae . Nat Prod Commun. 2014; ; 9 : :1193.–1210. 17. Wang J, Xu J, Xing G. Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway . Acta Biochim Biophys Sin (Shanghai). 2017; ; 49 : :771.–779. 18. Wang C, Wang Q, Li X, et al. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade . Biochem Biophys Res Commun. 2017; ; 483 : :197.–202. 19. Zhao D, Han W, Liu X, et al. MicroRNA-128 promotes apoptosis in lung cancer by directly targeting NIMA-related kinase 2 . Thorac Cancer. 2017; ; 8 : :304.–311. 20. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation . Cell. 2011; ; 144 : :646.–674. 21. Park S, Chapuis N, Tamburini J, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia . Haematologica. 2010; ; 95 : :819.–828. 22. Yoshikawa Y, Takano O, Kato I, et al. Ras inhibitors display an anti-metastatic effect by downregulation of lysyl oxidase through inhibition of the Ras-PI3K-Akt-HIF-1alpha pathway . Cancer Lett. 2017; ; 410 : :82.–91. 23. Yin K, Wang L, Zhang X, et al. Netrin-1 promotes gastric cancer cell proliferation and invasion via the receptor neogenin through PI3K/AKT signaling pathway . Oncotarget. 2017; ; 8 : :51177.–51189. 24. Hu B, Lv X, Gao F, et al. Downregulation of DEPTOR inhibits the proliferation, migration, and survival of osteosarcoma through PI3K/Akt/mTOR pathway . OncoTargets and therapy. 2017; ; 10 : :4379.–4391. 25. Wei Y, Wu S, Xu W, et al. Depleted aldehyde dehydrogenase 1A1 (ALDH1A1) reverses cisplatin resistance of human lung adenocarcinoma cell A549/DDP . Thorac Cancer. 2017; ; 8 : :26.–32. 26. Basho RK, Gilcrease M, Murthy RK, et al. Targeting the PI3K/AKT/mTOR Pathway for the Treatment of Mesenchymal Triple-Negative Breast Cancer: Evidence From a Phase 1 Trial of mTOR Inhibition in Combination With Liposomal Doxorubicin and Bevacizumab . JAMA Oncol. 2017; ; 3 : :509.–515. 27. Calero R, Morchon E, Martinez-Argudo I, Serrano R. Synergistic anti-tumor effect of 17AAG with the PI3K/mTOR inhibitor NVP-BEZ235 on human melanoma . Cancer Lett. 2017; ; 406 : :1.–11. 28. Choi EO, Cho EJ, Jeong JW, et al. Baicalein Inhibits the Migration and Invasion of B16F10 Mouse Melanoma Cells through Inactivation of the PI3K/Akt Signaling Pathway . Biomol Ther (Seoul). 2017; ; 25 : :213.–221. |