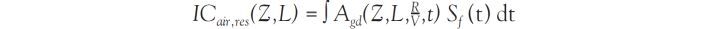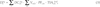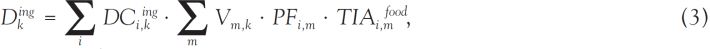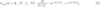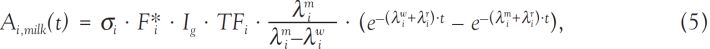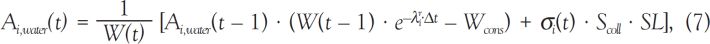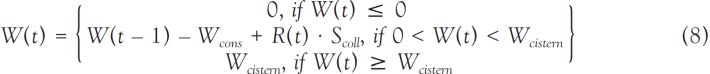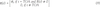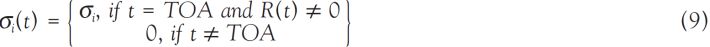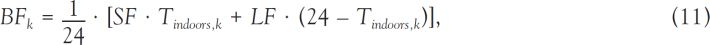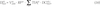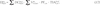2021
→ Aller vers ANALYSE→ Aller vers SYNTHESE
| Communications |
Dose assessment methods used to evaluate the radiation exposures
from nuclear testing in the atmosphere, with emphasis
on the tests conducted in French Polynesia
National Cancer Institute, Bethesda, MD, United States of America (retired)
Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, DHHS,
Bethesda, MD, United States of America
Types and characteristics of dose assessment
Information on average doses to large groups of people
 , 1962
, 1962 , 1966
, 1966 , 1969
, 1969 , 1972
, 1972 , 1977
, 1977 , 1982
, 1982 , 1993
, 1993 , 2000
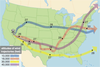
, 2000 ). The principal aims of the UNSCEAR reports was to estimate the average deposition densities of the most important radionuclides according to 10o latitude bands and the population-weighted doses over the entire populations of the northern and southern hemispheres, and of the entire world. As shown in Figure 1
). The principal aims of the UNSCEAR reports was to estimate the average deposition densities of the most important radionuclides according to 10o latitude bands and the population-weighted doses over the entire populations of the northern and southern hemispheres, and of the entire world. As shown in Figure 1 , the radioactive cloud produced by the explosion can be widely dispersed at the continental scale within a few days. The part of the radioactive cloud that is contained in the troposphere will circle the world, roughly in the same latitude band, in 20 to 30 days, while the part of the radioactive cloud that reaches the stratosphere will remain there during a year or two before descending to the ground.
, the radioactive cloud produced by the explosion can be widely dispersed at the continental scale within a few days. The part of the radioactive cloud that is contained in the troposphere will circle the world, roughly in the same latitude band, in 20 to 30 days, while the part of the radioactive cloud that reaches the stratosphere will remain there during a year or two before descending to the ground. . A summary of the temporal variation of the doses can be found in (Bouville et al., 2002
. A summary of the temporal variation of the doses can be found in (Bouville et al., 2002 ).
).
Tableau 1 Collective effective dose to the world population committed from atmospheric nuclear testing (based on UNSCEAR, 1993 )
)
|
Radionuclide
|
Half-life
|
Collective effective dose (1000 man Sv)
|
|||
|
External
|
Ingestion
|
Inhalation
|
Total
|
||
|
14C
|
5730 y
|
25800
|
2.6
|
25800
|
|
|
137Cs
|
30 y
|
1210
|
677
|
1.1
|
1890
|
|
90Sr
|
28.6 y
|
406
|
29
|
435
|
|
|
95Zr
|
64 d
|
272
|
6.1
|
278
|
|
|
106Ru
|
372 d
|
140
|
82
|
222
|
|
|
3H
|
12.3 y
|
176
|
13
|
189
|
|
|
54Mn
|
312 d
|
181
|
0.4
|
181
|
|
|
144Ce
|
285 d
|
44
|
122
|
166
|
|
|
131I
|
8.02 d
|
4.4
|
154
|
6.3
|
165
|
|
95Nb
|
35 d
|
129
|
2.6
|
132
|
|
|
125Sb
|
2.73 y
|
88
|
0.2
|
88
|
|
|
239Pu
|
24100 y
|
1.8
|
56
|
58
|
|
|
241Am
|
432 y
|
8.7
|
44
|
53
|
|
|
140Ba
|
12.8 d
|
49
|
0.81
|
0.66
|
50
|
|
103Ru
|
39 d
|
39
|
1.8
|
41
|
|
|
240Pu
|
6560 y
|
1.3
|
38
|
39
|
|
|
55Fe
|
2.74 y
|
26
|
0.06
|
26
|
|
|
241Pu
|
14.4 y
|
0.01
|
17
|
17
|
|
|
89Sr
|
51 d
|
4.5
|
6.0
|
11
|
|
|
91Y
|
58.5 d
|
8.9
|
8.9
|
||
|
141Ce
|
35 d
|
3.3
|
1.4
|
4.7
|
|
|
238Pu
|
86 y
|
0.003
|
2.3
|
2.3
|
|
|
Total
|
2160
|
27200
|
440
|
30000
|
|
Estimation of doses to critical groups
 ). The radiation measurements carried out in the local area (which typically extends to 200-300 km from the site of the explosion) are in part used to make sure that local populations were not subjected to excessive levels of radiation or that the maximally exposed critical groups received doses below the regulatory limits. These dose assessments are carried out using conservative assumptions and are not, as a rule, available in the open literature, although there are exceptions (see, for example, Ministère de la Défense, 2006
). The radiation measurements carried out in the local area (which typically extends to 200-300 km from the site of the explosion) are in part used to make sure that local populations were not subjected to excessive levels of radiation or that the maximally exposed critical groups received doses below the regulatory limits. These dose assessments are carried out using conservative assumptions and are not, as a rule, available in the open literature, although there are exceptions (see, for example, Ministère de la Défense, 2006 and Royal Commission into British Nuclear Tests in Australia, 1985
and Royal Commission into British Nuclear Tests in Australia, 1985 ). A review of the available information on the radiation doses to local populations near nuclear weapons test sites worldwide was published by Simon and Bouville (2002
). A review of the available information on the radiation doses to local populations near nuclear weapons test sites worldwide was published by Simon and Bouville (2002 ).
).Input data for use in epidemiologic studies of risk projection
 ). The methodology of dose assessment that was developed by ORERP (Anspaugh and Church, 1986
). The methodology of dose assessment that was developed by ORERP (Anspaugh and Church, 1986 ; Hicks, 1982
; Hicks, 1982 ; Whicker and Kirchner, 1987
; Whicker and Kirchner, 1987 ), as well as the data that were collected and processed, form the basis upon which the epidemiologic studies on fallout from nuclear weapons studies conducted in the U.S., either completed or ongoing, relies upon. In the area of risk projection, these studies, which were mandated by the U.S. Congress, include: (1) a National Cancer Institute (NCI) study of thyroid doses from 131I intakes, and resulting thyroid cancer, received by populations across the continental USA (NCI, 1997
), as well as the data that were collected and processed, form the basis upon which the epidemiologic studies on fallout from nuclear weapons studies conducted in the U.S., either completed or ongoing, relies upon. In the area of risk projection, these studies, which were mandated by the U.S. Congress, include: (1) a National Cancer Institute (NCI) study of thyroid doses from 131I intakes, and resulting thyroid cancer, received by populations across the continental USA (NCI, 1997 ), (2) a study jointly conducted by Centers for Disease Control and Prevention (CDC) and NCI on the feasibility to reliably estimate the health consequences to the American population from nuclear weapons tests conducted by the U.S. and other nations (DHHS, 2005
), (2) a study jointly conducted by Centers for Disease Control and Prevention (CDC) and NCI on the feasibility to reliably estimate the health consequences to the American population from nuclear weapons tests conducted by the U.S. and other nations (DHHS, 2005 ), (3) a study of radiation doses and cancer risks in the Marshall Islands from U.S. nuclear weapons tests (Simon et al., 2010a
), (3) a study of radiation doses and cancer risks in the Marshall Islands from U.S. nuclear weapons tests (Simon et al., 2010a ), and (4) a study to estimate radiation doses and cancer risks from radioactive fallout from the Trinity nuclear test (NCI, 2008
), and (4) a study to estimate radiation doses and cancer risks from radioactive fallout from the Trinity nuclear test (NCI, 2008 ). The doses calculated in these studies are as unbiased as possible and are for representative individuals with typical dietary, residential, and lifestyle habits. The dose values are then applied to the population groups corresponding to the representative individuals. An example of results obtained in this manner is shown in Table 2
). The doses calculated in these studies are as unbiased as possible and are for representative individuals with typical dietary, residential, and lifestyle habits. The dose values are then applied to the population groups corresponding to the representative individuals. An example of results obtained in this manner is shown in Table 2 .
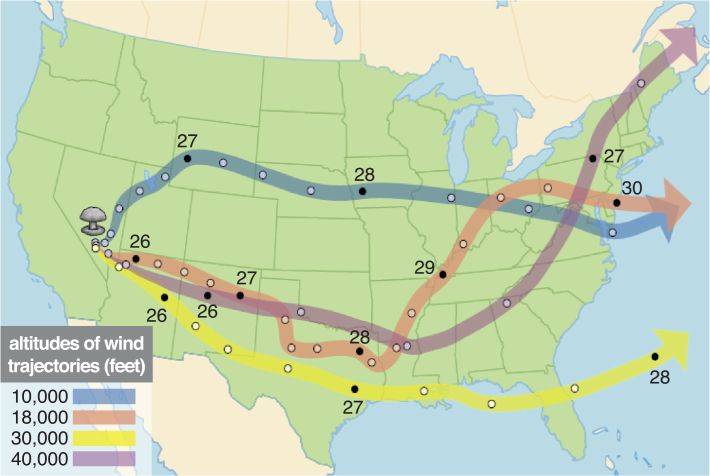
.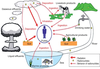 | Figure 2 Illustration of pathways of human exposure resulting from an atmospheric nuclear weapons test |
Tableau 2 Best estimates of cumulative acute internal and chronic internal doses (mGy) for four organs and of external dose (at all organs) to adults of four representative population groups (based on Simon et al., 2010a )
)
|
Organ / Mode of exposure
|
Population groups
|
|||
|
Majuro residents
|
Kwajalein residents
|
Utrik Community
|
Rongelap Community
|
|
|
Thyroid
| ||||
|
Acute internal
|
22
|
66
|
740
|
7600
|
|
Chronic internal
|
0.76
|
1.3
|
25
|
14
|
|
RBMa
| ||||
|
Acute internal
|
0.11
|
0.25
|
2.3
|
25
|
|
Chronic internal
|
0.98
|
1.7
|
33
|
17
|
|
Stomach wall
| ||||
|
Acute internal
|
0.32
|
1.1
|
16
|
530
|
|
Chronic internal
|
0.75
|
1.3
|
24
|
14
|
|
Colon
| ||||
|
Acute internal
|
4.4
|
12
|
180
|
2800
|
|
Chronic internal
|
0.99
|
1.7
|
32
|
17
|
|
Whole-body external
|
9.8
|
22
|
130
|
1600
|
a RBM: Red bone marrow.
Input data for use in analytical epidemiologic studies
 ), (2) the Utah thyroid cohort study, also related to the NTS tests (Till et al., 1995
), (2) the Utah thyroid cohort study, also related to the NTS tests (Till et al., 1995 ), (3) the Semipalatinsk cohort study, conducted jointly by U.S. and Russian investigators (Gordeev et al., 2006 a
), (3) the Semipalatinsk cohort study, conducted jointly by U.S. and Russian investigators (Gordeev et al., 2006 a and b
and b ), and (4) the French Polynesia thyroid case-control study (Drozdovitch et al., 2008
), and (4) the French Polynesia thyroid case-control study (Drozdovitch et al., 2008 , 2019
, 2019 , 2020a
, 2020a , 2020b
, 2020b ). In the analytical epidemiologic studies, information, as complete and reliable as possible, needs to be obtained on the residential, dietary, and lifestyle habits of all study subjects, generally through the use of a combination of individual interviews, focus groups, and available records. Because the individual dose estimates for analytical epidemiologic studies must be as unbiased as possible, it is necessary to use as many radiation measurements (exposure rates, radionuclide concentrations in air, water, foodstuffs, etc.) as possible. Fortunately, as will be seen later, large numbers of such measurements were made in French Polynesia at the time of the atmospheric tests (Coulon et al., 2009
). In the analytical epidemiologic studies, information, as complete and reliable as possible, needs to be obtained on the residential, dietary, and lifestyle habits of all study subjects, generally through the use of a combination of individual interviews, focus groups, and available records. Because the individual dose estimates for analytical epidemiologic studies must be as unbiased as possible, it is necessary to use as many radiation measurements (exposure rates, radionuclide concentrations in air, water, foodstuffs, etc.) as possible. Fortunately, as will be seen later, large numbers of such measurements were made in French Polynesia at the time of the atmospheric tests (Coulon et al., 2009 ).
).
Tableau 3 Summary of active marrow doses (mGy) for the 6507 study subjects of the Utah leukemia case-control study (based on Simon et al., 1995 )
)
|
Cases
|
Controls
|
Overall
|
|
|
Mean
|
2.9
|
2.7
|
2.8
|
|
Median
|
3.2
|
3.1
|
3.2
|
|
Mode
|
3.4
|
3.4
|
3.4
|
|
Minimum
|
0.0
|
0.0
|
0.0
|
|
Maximum
|
26.0
|
29.0
|
29.0
|
|
Variance
|
0.64
|
0.48
|
0.51
|
Validation of the dose estimates
 ), but it does not seem to have been applied to any other fallout study related to nuclear weapons tests. In the NTS study conducted by NCI (1997
), but it does not seem to have been applied to any other fallout study related to nuclear weapons tests. In the NTS study conducted by NCI (1997 ), the 131I concentrations measured in urine were used to indirectly validate the thyroid doses.
), the 131I concentrations measured in urine were used to indirectly validate the thyroid doses.Uncertainties in the dose estimates
 ). Until recent years, the evaluation of the uncertainties consisted of numerical simulations in which variability and lack-of-knowledge uncertainties were combined in Monte-Carlo simulations. In that method, probability density distributions are assigned to the parameter values that are deemed to have a substantial influence on the dose estimate and multiple realizations of individual doses are estimated (NCRP, 1996
). Until recent years, the evaluation of the uncertainties consisted of numerical simulations in which variability and lack-of-knowledge uncertainties were combined in Monte-Carlo simulations. In that method, probability density distributions are assigned to the parameter values that are deemed to have a substantial influence on the dose estimate and multiple realizations of individual doses are estimated (NCRP, 1996 ). The primary limitation of many such simulations is that shared errors and intra-individual correlations are not accounted for. A more sophisticated method, the two-dimensional Monte-Carlo procedure, was used in the Semipalatinsk study to separate and distinguish between the shared and the unshared components (Land et al., 2015
). The primary limitation of many such simulations is that shared errors and intra-individual correlations are not accounted for. A more sophisticated method, the two-dimensional Monte-Carlo procedure, was used in the Semipalatinsk study to separate and distinguish between the shared and the unshared components (Land et al., 2015 ; Simon et al., 2015
; Simon et al., 2015 ). In most studies, uncertainties were evaluated in a subjective manner, if at all.
). In most studies, uncertainties were evaluated in a subjective manner, if at all.Methods of dose assessment used for the U.S. and Russian tests
Estimation of external doses
Estimation of the normalized outdoor exposure rates
 ). This is the equation that was typically used for the dose assessments related to the Russian tests (Gordeev et al., 2006a
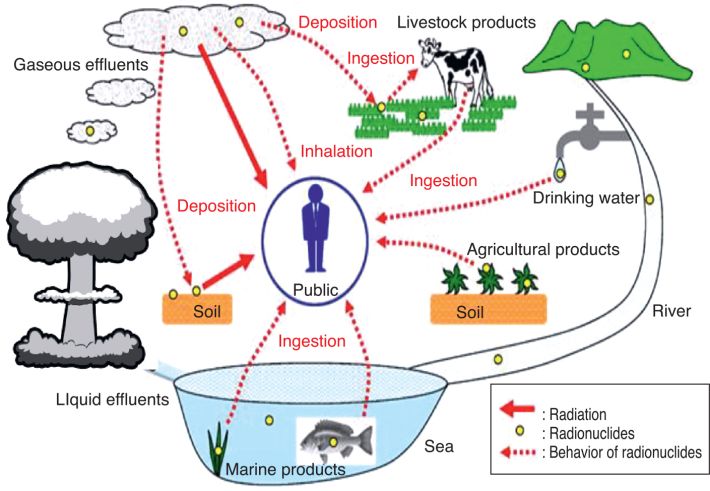
). This is the equation that was typically used for the dose assessments related to the Russian tests (Gordeev et al., 2006a ). In the United States, the temporal variation of the exposure rates was established for all important tests as a 10-component multi-exponential function, which is rather complex but can be applied to any time after the explosion. For a given degree of fractionation between refractory and volatile radionuclides (R/V fractionation ratio), there is little variation from one test to another (see Figure 3
). In the United States, the temporal variation of the exposure rates was established for all important tests as a 10-component multi-exponential function, which is rather complex but can be applied to any time after the explosion. For a given degree of fractionation between refractory and volatile radionuclides (R/V fractionation ratio), there is little variation from one test to another (see Figure 3 ).
).Estimation of the total exposure
 , taking the fractionation ratio R/V into account. The fractionation ratio reflects the fact that particles of all sizes are in the radioactive cloud. The large particles, with sizes > 50 μm, are enriched with refractory radionuclides, whereas the small particles, with sizes < 50 μm, are enriched with volatile radionuclides. Because the large particles deposit more quickly than the small particles, meaning that they reach the ground at smaller distances from the site of the explosion, the value of R/V decreases as the distance from the site of the explosion increases. The value of R/V, which usually is in the range from 0.5 to 3.0, is estimated to vary as a function of TOA/Tcr, where the critical time Tcr is the length of time since detonation for all particles > 50 μm to be deposited (Beck et al., 2010
, taking the fractionation ratio R/V into account. The fractionation ratio reflects the fact that particles of all sizes are in the radioactive cloud. The large particles, with sizes > 50 μm, are enriched with refractory radionuclides, whereas the small particles, with sizes < 50 μm, are enriched with volatile radionuclides. Because the large particles deposit more quickly than the small particles, meaning that they reach the ground at smaller distances from the site of the explosion, the value of R/V decreases as the distance from the site of the explosion increases. The value of R/V, which usually is in the range from 0.5 to 3.0, is estimated to vary as a function of TOA/Tcr, where the critical time Tcr is the length of time since detonation for all particles > 50 μm to be deposited (Beck et al., 2010 ). For locations where TOA > Tcr, all deposited particles are smaller than 50 μm and R/V = 0.5.
). For locations where TOA > Tcr, all deposited particles are smaller than 50 μm and R/V = 0.5.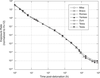 | Figure 3 Variation of the exposure rate with time for several atmospheric tests for a fractionation ratio (R/V) of 0.5 (Bouville et al., 2010 ) ) |
 ), so that almost the totality of the exposure is obtained during the first year after the detonation.
), so that almost the totality of the exposure is obtained during the first year after the detonation.Estimation of the organ and tissue doses
 ). This factor varies with the energy of the gamma ray and with the orientation with respect to radiation incidence, as well as with the organ or tissue that is considered and with the anthropomorphic characteristics of the person. Because there is little difference between the values of the conversion factor from an organ to another for gamma rays of a few hundred keV that are typical for fission products, the same value can be used for adults for all organs and tissues usually considered in fallout studies. However, calculations using anthropomorphic phantoms of different ages indicate that slightly higher values are obtained for younger ages (Jacob et al., 1990
). This factor varies with the energy of the gamma ray and with the orientation with respect to radiation incidence, as well as with the organ or tissue that is considered and with the anthropomorphic characteristics of the person. Because there is little difference between the values of the conversion factor from an organ to another for gamma rays of a few hundred keV that are typical for fission products, the same value can be used for adults for all organs and tissues usually considered in fallout studies. However, calculations using anthropomorphic phantoms of different ages indicate that slightly higher values are obtained for younger ages (Jacob et al., 1990 ). Based on those calculations, the conversion factors for younger (< 3 y, including in utero) and older (3 through 14 y) children were derived in the Marshall Islands study by multiplying the adult conversion factors by 1.3 and 1.2, respectively (Bouville et al., 2010
). Based on those calculations, the conversion factors for younger (< 3 y, including in utero) and older (3 through 14 y) children were derived in the Marshall Islands study by multiplying the adult conversion factors by 1.3 and 1.2, respectively (Bouville et al., 2010 ). Finally, the calculation of the outdoor dose must take the fraction of time spent outdoors into account. If it is assumed to be 0.2 (UNSCEAR, 1993
). Finally, the calculation of the outdoor dose must take the fraction of time spent outdoors into account. If it is assumed to be 0.2 (UNSCEAR, 1993 ), the overall conversion factor from outdoor exposure to tissue dose is 8.75 10-3 (Gy R-1) × 0.75 (Gy Gy-1) × 0.2 = 1.3 10-3 Gy R-1 for representative adults. For specific individuals, the value of the fraction of time spent out of doors must be obtained from individual interviews or derived from focus groups or interviews of experts.
), the overall conversion factor from outdoor exposure to tissue dose is 8.75 10-3 (Gy R-1) × 0.75 (Gy Gy-1) × 0.2 = 1.3 10-3 Gy R-1 for representative adults. For specific individuals, the value of the fraction of time spent out of doors must be obtained from individual interviews or derived from focus groups or interviews of experts. ), the overall conversion factor from indoor exposure to tissue dose is 8.75×10-3 (Gy R-1) × 0.75 (Gy Gy-1) × 0.8 × 0.2 = 1.05×10-3 Gy R-1 for representative adults. For specific individuals, the value of the fraction of time spent indoors must be obtained from individual interviews or derived from focus groups or interviews of experts; the value of the shielding factor is ideally obtained from measurements. In the absence of measurements, literature values (for example, Glasstone and Dolan, 1977
), the overall conversion factor from indoor exposure to tissue dose is 8.75×10-3 (Gy R-1) × 0.75 (Gy Gy-1) × 0.8 × 0.2 = 1.05×10-3 Gy R-1 for representative adults. For specific individuals, the value of the fraction of time spent indoors must be obtained from individual interviews or derived from focus groups or interviews of experts; the value of the shielding factor is ideally obtained from measurements. In the absence of measurements, literature values (for example, Glasstone and Dolan, 1977 ) are used.
) are used.Estimation of internal doses
 ), as the doses from acute intakes of radionuclides are derived from historical measurements of 131I in pooled samples of urine collected from adults about 2 weeks after the Bravo test (Harris et al., 2010
), as the doses from acute intakes of radionuclides are derived from historical measurements of 131I in pooled samples of urine collected from adults about 2 weeks after the Bravo test (Harris et al., 2010 ) and the doses from intakes of long-lived radionuclides are based on measurements of whole-body activity of 137Cs, 60Co, and 65Zn (Lessard et al., 1984
) and the doses from intakes of long-lived radionuclides are based on measurements of whole-body activity of 137Cs, 60Co, and 65Zn (Lessard et al., 1984 ). For the other U.S. tests and for the Russian tests, bioassay data are either non-existent or limited to a small of persons (see, for example NCI, 1997
). For the other U.S. tests and for the Russian tests, bioassay data are either non-existent or limited to a small of persons (see, for example NCI, 1997 ). This is true as well for environmental radiation data. In most cases, the assessment of internal doses related to U.S. and Russian tests is based on models of environmental transfer from the activity deposited on the ground to the radionuclide concentrations in air, water, and foodstuffs; it generally consists of 5 steps: (1) estimation of the ground deposition densities (Bq m-2), (2) estimation of radionuclide concentrations in the vegetation and in soil (Bq kg-1), (3) estimation of radionuclide concentrations in air (Bq m-3), water (Bq L-1), milk (Bq L-1), plants, animals and animal products (Bq kg-1), (4) estimation of internal doses from inhalation (Gy), and (5) estimation of internal doses from ingestion (Gy).
). This is true as well for environmental radiation data. In most cases, the assessment of internal doses related to U.S. and Russian tests is based on models of environmental transfer from the activity deposited on the ground to the radionuclide concentrations in air, water, and foodstuffs; it generally consists of 5 steps: (1) estimation of the ground deposition densities (Bq m-2), (2) estimation of radionuclide concentrations in the vegetation and in soil (Bq kg-1), (3) estimation of radionuclide concentrations in air (Bq m-3), water (Bq L-1), milk (Bq L-1), plants, animals and animal products (Bq kg-1), (4) estimation of internal doses from inhalation (Gy), and (5) estimation of internal doses from ingestion (Gy).Estimation of the ground deposition densities of each radionuclide
 ) provide not only the variation of the exposure rate with time after the detonation for values of R/V of 1.0 and 0.5, but also the corresponding ground deposition densities of a large range of radionuclides. Beck et al. (2010
) provide not only the variation of the exposure rate with time after the detonation for values of R/V of 1.0 and 0.5, but also the corresponding ground deposition densities of a large range of radionuclides. Beck et al. (2010 ) extended these calculations to other values of R/V appropriate to fallout near the site of the explosion.
) extended these calculations to other values of R/V appropriate to fallout near the site of the explosion.Estimation of vegetation and soil radionuclide concentrations
 ).
).Estimation of radionuclide concentrations in air, water, and foodstuffs
 ; Thiessen and Hoffman, 2018
; Thiessen and Hoffman, 2018 ; Whicker and Kirchner, 1987
; Whicker and Kirchner, 1987 ).
).Estimation of internal doses from inhalation
 ) to be:
) to be: ; Maxwell and Anspaugh, 2011
; Maxwell and Anspaugh, 2011 ):
):Estimation of internal doses from ingestion
 ; Ng et al., 1990
; Ng et al., 1990 ). For specific individuals, the food consumption rates of the study subjects must be obtained from individual interviews or derived from focus groups or interviews of experts (Schwerin et al., 2010
). For specific individuals, the food consumption rates of the study subjects must be obtained from individual interviews or derived from focus groups or interviews of experts (Schwerin et al., 2010 ; Drozdovitch et al., 2011
; Drozdovitch et al., 2011 ).
).Methods used for the tests conducted in French Polynesia
 ; Bataille and Revol, 2002
; Bataille and Revol, 2002 ). The nuclear test sites were two atolls, Muruora and Fangataufa, located in the southeastern part of Tuamotu-Gambier archipelago at about 1150 km from Tahiti, the most populated island in French Polynesia.
). The nuclear test sites were two atolls, Muruora and Fangataufa, located in the southeastern part of Tuamotu-Gambier archipelago at about 1150 km from Tahiti, the most populated island in French Polynesia. ; IAEA, 2009-2010
; IAEA, 2009-2010 ). In addition, 25 campaigns of anthropogammametry measurements were conducted among the populations of the islands close to the nuclear sites; unfortunately, the results, expressed in terms of triage index, cannot be used for dose assessment purposes as no additional information is available.
). In addition, 25 campaigns of anthropogammametry measurements were conducted among the populations of the islands close to the nuclear sites; unfortunately, the results, expressed in terms of triage index, cannot be used for dose assessment purposes as no additional information is available. ; DSND, 2006a
; DSND, 2006a , 2006b
, 2006b ) and by Inserm in a study of thyroid cancer in French Polynesia (Drozdovitch et al., 2008
) and by Inserm in a study of thyroid cancer in French Polynesia (Drozdovitch et al., 2008 , 2019
, 2019 , 2020a
, 2020a , 2020b
, 2020b ) are presented in turn.
) are presented in turn.Dose assessment by the French authorities
 ). The main purpose of the dose assessments was to make sure that the dose levels were below the regulatory limits.
). The main purpose of the dose assessments was to make sure that the dose levels were below the regulatory limits.Assessment of the external doses
• Immersion dose during the passage of the radioactive cloud
 ). The time-integrated concentration in air, expressed in Bq s m-3, was then obtained using a deposition velocity, the value of which varied according to estimated TOA at the location considered and the occurrence, or not, of rain. Deposition velocities ranging from 10-3 to 10-1 m s-1 were used for TOAs shorter than one day. The final step of the calculation of the immersion dose consisted in applying an appropriate effective dose coefficient, expressed in Sv per Bq s m-3, to each radionuclide (Eckerman and Ryman, 1993
). The time-integrated concentration in air, expressed in Bq s m-3, was then obtained using a deposition velocity, the value of which varied according to estimated TOA at the location considered and the occurrence, or not, of rain. Deposition velocities ranging from 10-3 to 10-1 m s-1 were used for TOAs shorter than one day. The final step of the calculation of the immersion dose consisted in applying an appropriate effective dose coefficient, expressed in Sv per Bq s m-3, to each radionuclide (Eckerman and Ryman, 1993 ) and in summing the results over the 70 radionuclides. The reduction of the dose due to shielding while indoors was taken into account, using a protection factor of 0.5 if the radioactive cloud arrived during the night or while the population was sheltered. The dependence of the dose with age was not taken into account.
) and in summing the results over the 70 radionuclides. The reduction of the dose due to shielding while indoors was taken into account, using a protection factor of 0.5 if the radioactive cloud arrived during the night or while the population was sheltered. The dependence of the dose with age was not taken into account.• External dose resulting from ground deposition of fallout
 ). Integrating over the time of exposure, taking radioactive decay into account, yields the external effective dose due to the radionuclide under consideration. Summation over the approximately 70 radionuclides leads to the total external effective dose due to the ground deposited activity. Modifying factors were applied to this result: (1) a reduction factor of 2/3 based on the assumption that people spent part of their time in the contaminated area, and (2) when the radioactive cloud arrived during the night, it was assumed that the populations, being indoors, were not exposed during the first 6 hours after TOA.
). Integrating over the time of exposure, taking radioactive decay into account, yields the external effective dose due to the radionuclide under consideration. Summation over the approximately 70 radionuclides leads to the total external effective dose due to the ground deposited activity. Modifying factors were applied to this result: (1) a reduction factor of 2/3 based on the assumption that people spent part of their time in the contaminated area, and (2) when the radioactive cloud arrived during the night, it was assumed that the populations, being indoors, were not exposed during the first 6 hours after TOA. ; Bouville and Beck, 2000
; Bouville and Beck, 2000 ).
).• Internal dose resulting from inhalation of radioactive materials
• Internal dose resulting from ingestion of water, milk, and foodstuffs
 ; Grouzelle et al., 1985
; Grouzelle et al., 1985 ). Only locally produced foodstuffs were considered. The consumption rates of children were derived from the consumption rates of adults.
). Only locally produced foodstuffs were considered. The consumption rates of children were derived from the consumption rates of adults. , 1996a
, 1996a , 1996b
, 1996b ).
).• Dose estimates
 and 5
and 5 at the most exposed locations for each of the exposure pathways taken into consideration.
at the most exposed locations for each of the exposure pathways taken into consideration. and 5
and 5 show that the consumption of water and seafood were important exposure pathways in the atolls and islands close to the test sites, where the populations were small and the diet was limited to a few staples. In Tahiti, where more than half of the total French Polynesian population resided, there was a large variety of food products, including cow’s milk, and the consumption of water played a relatively minor role.
show that the consumption of water and seafood were important exposure pathways in the atolls and islands close to the test sites, where the populations were small and the diet was limited to a few staples. In Tahiti, where more than half of the total French Polynesian population resided, there was a large variety of food products, including cow’s milk, and the consumption of water played a relatively minor role.
Tableau 4 Estimated thyroid doses to 1-2 y old children (mGy), calculated by the French authorities for the 6 tests with significant fallout (based on Ministère de la Défense, 2006 )
)
|
Test Name
|
Dose location
|
Ext. cloud
|
Ext, dep.
|
Inhala-
tion
|
Ingestion food
|
Ingestion water
|
Total
|
Major pathway
|
|
Aldebaran
|
Gambier
|
0.02-0.2
|
2.9
|
3-30
|
1.3-42
|
0-6
|
7.2-81
|
Inhalation, water
|
|
Rigel
|
Tureia
|
Small
|
0.05
|
0.03
|
0.06-1.15
|
0.55-0.88
|
0.65-2
|
Water
|
|
Rigel
|
Gambier
|
Small
|
0.019
|
0.011
|
0.15-0.51
|
4.4-7.3
|
4.6-7.8
|
Water
|
|
Arcturus
|
Tureia
|
Small
|
0.7
|
0.2-1.4
|
0.7-34.8
|
1.24
|
2.2-38
|
Seafood
|
|
Encelade
|
Tureia
|
Small
|
1.1
|
0.14-0.8
|
0.71-4.6
|
3.0-21.1
|
4.9-28
|
Water
|
|
Phoebé
|
Gambier
|
Small
|
0.11
|
0.01-0.04
|
0.52-9.6
|
4.3-88.2
|
4.8-98
|
Water
|
|
Centaure
|
Pirae
|
0.002
|
0.053
|
0.57
|
13
|
0.6
|
14
|
Milk, seafood, plants
|
|
Centaure
|
Hitiaa
|
0.025
|
1.2
|
6.4
|
41
|
1.3
|
50
|
Milk, seafood, plants
|
|
Centaure
|
Taravao
|
0.09
|
1.1
|
24
|
15.4
|
0.22
|
40
|
Inhalation, milk, seafood, plants
|
Tableau 5 Estimated effective doses to adults (mSv), calculated by the French authorities for the 6 tests with significant fallout (based on Ministère de la Défense, 2006 )
)
|
Test name
|
Dose location
|
Ext. cloud
|
Ext, dep.
|
Inhalation
|
Ingestion food
|
Ingestion water
|
Total
|
Major pathway
|
|
Aldebaran
|
Gambier
|
0.02-0.2
|
2.9
|
0.1-1.2
|
0.09-2.2
|
0-0.12
|
3.1-6.6
|
External
|
|
Rigel
|
Tureia
|
Small
|
0.05
|
0.002
|
0.002-0.07
|
0.01-0.02
|
0.06-0.1
|
External
|
|
Rigel
|
Gambier
|
Small
|
0.019
|
Small
|
0.01-0.04
|
0.1-0.17
|
0.1-0.2
|
Water
|
|
Arcturus
|
Tureia
|
Small
|
0.7
|
0.01-0.07
|
0.04-2.4
|
0.03
|
0.8-3.2
|
Seafood
|
|
Encelade
|
Tureia
|
Small
|
1.1
|
0.01
|
0.06-0.31
|
0.06-0.5
|
1.2-1.9
|
External
|
|
Phoebé
|
Gambier
|
Small
|
0.11
|
Small
|
0.03-0.66
|
0.1-1.8
|
0.2-2.6
|
Water
|
|
Centaure
|
Pirae
|
0.002
|
0.053
|
0.046
|
0.34
|
0.016
|
0.5
|
Food
|
|
Centaure
|
Hitiaa
|
0.025
|
1.2
|
0.52
|
0.82
|
0.03
|
2.6
|
External
|
|
Centaure
|
Taravao
|
0.09
|
1.1
|
1.9
|
0.46
|
0.0045
|
3.6
|
External
|
Dose assessment for the Inserm study of thyroid cancer
in French Polynesia
 ), a population-based case-control study of thyroid cancer was performed. The study consisted of two phases. Phase I included all alive cases of thyroid cancer developed between 1985 and 2003 in persons who were children, adolescents, and young adults at the time of atmospheric nuclear testing. Epidemiological aspects of Phase I and estimates of risk of thyroid cancer were published by de Vathaire et al. (2010). Overall, 602 subjects, both cases and controls, were included in the risk analysis, which was performed using thyroid doses calculated in 2008 by means of the “Thyroid Dosimetry 2008 system” (TD08) (Drozdovitch et al., 2008
), a population-based case-control study of thyroid cancer was performed. The study consisted of two phases. Phase I included all alive cases of thyroid cancer developed between 1985 and 2003 in persons who were children, adolescents, and young adults at the time of atmospheric nuclear testing. Epidemiological aspects of Phase I and estimates of risk of thyroid cancer were published by de Vathaire et al. (2010). Overall, 602 subjects, both cases and controls, were included in the risk analysis, which was performed using thyroid doses calculated in 2008 by means of the “Thyroid Dosimetry 2008 system” (TD08) (Drozdovitch et al., 2008 ). In 2014-2017, Inserm undertook Phase II of the epidemiological study, including 348 additional subjects, thus resulting in a total of 950 subjects. Because of deficiencies in TD08, mainly related to limitations in the input data, the dosimetry system was improved for the assessment of thyroid doses for all subjects of the epidemiologic study. Unit 605 of Inserm (currently Unit 1018) coordinated the case-control study.
). In 2014-2017, Inserm undertook Phase II of the epidemiological study, including 348 additional subjects, thus resulting in a total of 950 subjects. Because of deficiencies in TD08, mainly related to limitations in the input data, the dosimetry system was improved for the assessment of thyroid doses for all subjects of the epidemiologic study. Unit 605 of Inserm (currently Unit 1018) coordinated the case-control study. ). The radiation dose to the thyroid gland had to be evaluated for each study subject. However, the following limitations of TD08 were recognized:
). The radiation dose to the thyroid gland had to be evaluated for each study subject. However, the following limitations of TD08 were recognized: ).
). , 1969
, 1969 , 1971
, 1971 -1975
-1975 ) by the French Government after each series of tests. However, the results of radiation monitoring were reported only for 9 islands and atolls after some tests.
) by the French Government after each series of tests. However, the results of radiation monitoring were reported only for 9 islands and atolls after some tests.Behavior and food consumption pattern of the French Polynesian population in the 1960s-1970s
 ). The focus group discussions and key informant interviews are retrospective data collection strategies that provide more reliable recall than individual subject interviews. Low validity and reproducibility of data on recalled individual diet are typically characterized for recollections exceeding 10 years (Willett, 1998
). The focus group discussions and key informant interviews are retrospective data collection strategies that provide more reliable recall than individual subject interviews. Low validity and reproducibility of data on recalled individual diet are typically characterized for recollections exceeding 10 years (Willett, 1998 ) and recall of diet in distant past is strongly influenced by present dietary habits (Rohan and Potter, 1984
) and recall of diet in distant past is strongly influenced by present dietary habits (Rohan and Potter, 1984 ). Focus group discussion helps to stimulate recall about lifestyle questions and overcome low reproducibility in providing information. Interaction of focus group participants is a unique and compelling feature where participants share their experiences to provide “true” group consensus data as well as the reasons for differences among participants (Kitzinger, 1995
). Focus group discussion helps to stimulate recall about lifestyle questions and overcome low reproducibility in providing information. Interaction of focus group participants is a unique and compelling feature where participants share their experiences to provide “true” group consensus data as well as the reasons for differences among participants (Kitzinger, 1995 ). However, we observed during the study that individual opinion may be inflected or influenced by group consensus; this may be a limitation of focus group strategy. The focus-group methodology was successfully used to collect quantitative and qualitative data on lifestyle and occupational habits for the purposes of retrospective dose reconstruction for radiation epidemiology studies of population exposed in 1949-1962 to fallout from Semipalatinsk nuclear test site in Kazakhstan population (Drozdovitch et al., 2011
). However, we observed during the study that individual opinion may be inflected or influenced by group consensus; this may be a limitation of focus group strategy. The focus-group methodology was successfully used to collect quantitative and qualitative data on lifestyle and occupational habits for the purposes of retrospective dose reconstruction for radiation epidemiology studies of population exposed in 1949-1962 to fallout from Semipalatinsk nuclear test site in Kazakhstan population (Drozdovitch et al., 2011 ; Schwerin et al., 2010
; Schwerin et al., 2010 ) and nuclear medicine technologists who diagnostic radioisotope procedures in the 1950s-mid 1970s (Drozdovitch et al., 2014
) and nuclear medicine technologists who diagnostic radioisotope procedures in the 1950s-mid 1970s (Drozdovitch et al., 2014 ).
).Focus groups
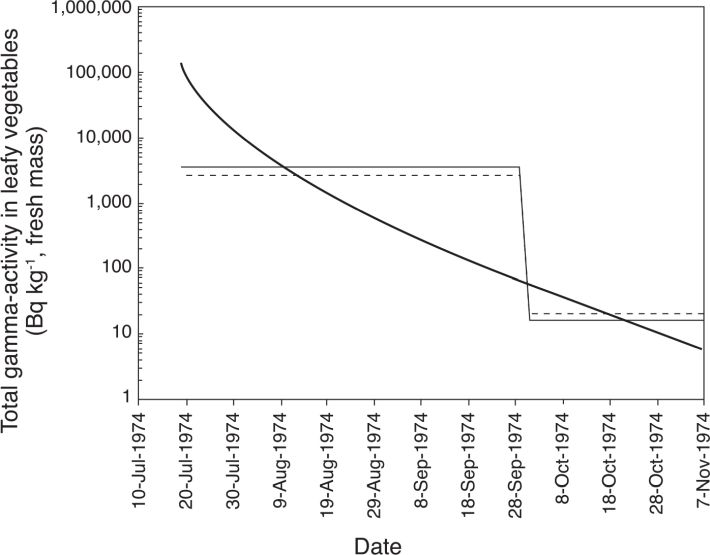
 shows, as example of data collected during the focus groups, daily consumption by children of different ages in mid 1960s – mid 1970s of fresh cow’s milk, leafy vegetables and fâfâ that were the major sources of 131I intake with food.
shows, as example of data collected during the focus groups, daily consumption by children of different ages in mid 1960s – mid 1970s of fresh cow’s milk, leafy vegetables and fâfâ that were the major sources of 131I intake with food.
Tableau 6 Daily consumptiona,b (g(mL) d-1) of foodstuffs by children of different ages in mid 1960s – mid 1970s (Drozdovitch et al., 2019 )
)
|
Foodstuff
|
Archipelago / Island
|
Age, y
|
||||
|
< 1
|
1-3
|
4-6
|
7-14
|
15-21
|
||
|
Fresh cow’s milk
|
Tahiti
|
–
|
371±70
|
321±53
|
213±25
|
314±62
|
|
Leafy vegetables
|
Tahiti
|
–
|
31±3.7
|
36±3.2
|
61±7.9
|
79±14
|
|
Society
|
109±34
|
147±27
|
210c
|
7
|
121±55
|
|
|
Tuamotu
|
–
|
–
|
30
|
110±32
|
96±27
|
|
|
Gambier
|
–
|
60
|
76±5.6
|
62±14
|
93±40
|
|
|
Marquises
|
–
|
5.2±1.4
|
88±14
|
260
|
92±31
|
|
|
Australes
|
–
|
48±7.3
|
73±17
|
110±44
|
86±15
|
|
|
Fâfâ
|
Tahiti
|
–
|
2.9
|
38±12
|
54±8.0
|
61±8.2
|
|
Society
|
12±1.8
|
18±1.2
|
11±1.6
|
69±18
|
77±8.8
|
|
|
Gambier
|
–
|
–
|
21±5.9
|
7.8±1.4
|
14±3.1
|
|
|
Marquises
|
–
|
8.5±2.9
|
2.7
|
–
|
61±30
|
|
|
Australes
|
104±25
|
107±13
|
100±27
|
130±19
|
160±32
|
|
a Arithmetic mean ± standard error of mean among children for whom consumption of cow’s milk, leafy vegetables and fâfâ was reported.
b Locally produced food unless otherwise indicated.
c For values printed in italic, all focus group participants reported the same consumption rates for their children.
Key informant interviews
 ).
).Reconstruction of ground deposition of radionuclides in French Polynesia resulting from atmospheric nuclear weapons tests at Mururoa and Fangataufa atolls
Radiation monitoring
 ; Ministère de la Défense, 2006
; Ministère de la Défense, 2006 ).
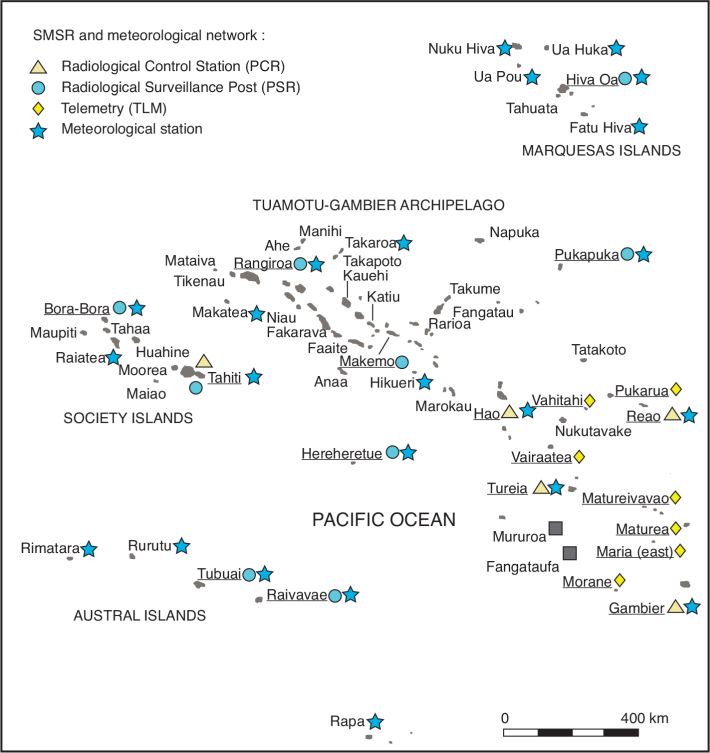
). shows the locations of PCRs, PSRs and TLMs in French Polynesia during the time period of the atmospheric nuclear tests. It should be noted that the numbers of PCR and PSR varied from year to year. In addition, measurements of total beta- concentration in air were performed in 1966 in Moorea, Raiatea (Society Islands) and in Anaa, Makemo, Hikueri, Takaroa (Tuamotu); however, these locations were not included in the SMSR network in later years and are not shown on Figure 4
shows the locations of PCRs, PSRs and TLMs in French Polynesia during the time period of the atmospheric nuclear tests. It should be noted that the numbers of PCR and PSR varied from year to year. In addition, measurements of total beta- concentration in air were performed in 1966 in Moorea, Raiatea (Society Islands) and in Anaa, Makemo, Hikueri, Takaroa (Tuamotu); however, these locations were not included in the SMSR network in later years and are not shown on Figure 4 . In addition to the SMSR reports, meteorological information, namely daily precipitation and wind speed and direction, was available from Météo France; the location of the 23 meteorological stations is also shown on Figure 4
. In addition to the SMSR reports, meteorological information, namely daily precipitation and wind speed and direction, was available from Météo France; the location of the 23 meteorological stations is also shown on Figure 4 .
.
 , 1972
, 1972 , 1973
, 1973 ) were considered.
) were considered. -1975) reports in comparison with those available in UNSCEAR reports: 7,526 vs 439 for total beta-concentration in filtered air, 251 vs 0 for ground deposition density, 339 vs 2 for exposure rate, respectively. The numbers of measurements of 131I activity concentration in cow’s milk were found to be similar in the SMCB reports (SMCB, 1970
-1975) reports in comparison with those available in UNSCEAR reports: 7,526 vs 439 for total beta-concentration in filtered air, 251 vs 0 for ground deposition density, 339 vs 2 for exposure rate, respectively. The numbers of measurements of 131I activity concentration in cow’s milk were found to be similar in the SMCB reports (SMCB, 1970

 , 1972
, 1972 , 1973
, 1973
 ) and in the reports to UNSCEAR.
) and in the reports to UNSCEAR. | Figure 4 Locations of SMSR network (underlined names of islands and atolls) and meteorological stations in French Polynesia in 1966-1974 (Drozdovitch et al., 2020a ) ) |
Estimation of the time of arrival of fallout (TOA)
 -1975), the reports to UNSCEAR (Republic of France, 1967
-1975), the reports to UNSCEAR (Republic of France, 1967 , 1969
, 1969 , 1971
, 1971 -1975
-1975 ), or the report from the Ministère de la Défense (2006
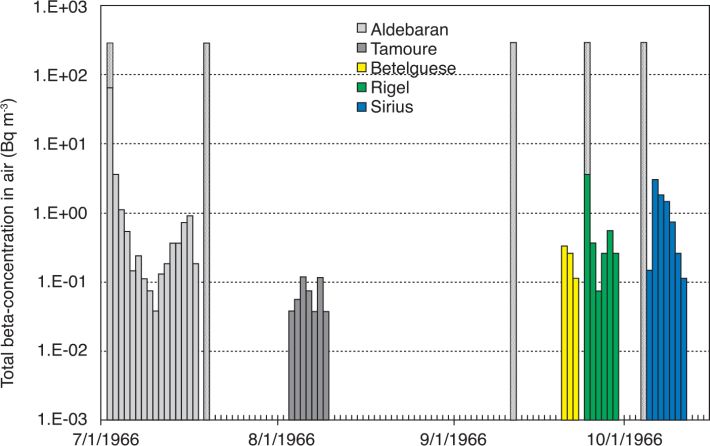
), or the report from the Ministère de la Défense (2006 ). When the TOA values were not available in those reports, they were estimated from the results of measurements of daily total beta-concentration in air. Because of the horizontal and vertical wind shear, the radioactive clouds produced by the nuclear weapons tests usually followed different trajectories during the atmospheric transport over the large territory of French Polynesia; and there were many cases where “secondary fallout” extended over several days and where there were not one, but several waves of ground deposition. Figure 5
). When the TOA values were not available in those reports, they were estimated from the results of measurements of daily total beta-concentration in air. Because of the horizontal and vertical wind shear, the radioactive clouds produced by the nuclear weapons tests usually followed different trajectories during the atmospheric transport over the large territory of French Polynesia; and there were many cases where “secondary fallout” extended over several days and where there were not one, but several waves of ground deposition. Figure 5 shows, for example, the variation with time of the daily total beta-concentration in air measured in Gambier Islands after the tests conducted in 1966: one part of the radioactive cloud from test Aldébaran (conducted on 2 July 1966) led according to data from SMSR (1966a
shows, for example, the variation with time of the daily total beta-concentration in air measured in Gambier Islands after the tests conducted in 1966: one part of the radioactive cloud from test Aldébaran (conducted on 2 July 1966) led according to data from SMSR (1966a ) to direct fallout that reached Gambier at TOA = H+10h45 (“H” denotes time of detonation). Secondary fallout after test Aldébaran started at Gambier at H+9 d, and maximal concentration of total beta-concentration in air was reached on days 13 and 14 after the test. TOA for secondary fallout was taken to occur during the time of maximal concentration and to be H+13 d. The same considerations were applied for TOA after test Rigel (conducted on 24 September 1966): TOA for direct fallout was taken as H+12 h according to SMSR (1966b
) to direct fallout that reached Gambier at TOA = H+10h45 (“H” denotes time of detonation). Secondary fallout after test Aldébaran started at Gambier at H+9 d, and maximal concentration of total beta-concentration in air was reached on days 13 and 14 after the test. TOA for secondary fallout was taken to occur during the time of maximal concentration and to be H+13 d. The same considerations were applied for TOA after test Rigel (conducted on 24 September 1966): TOA for direct fallout was taken as H+12 h according to SMSR (1966b ) and TOA of H+4 d for secondary fallout was derived from the temporal variation of the results of measurements of daily total beta-concentration in air (Figure 5
) and TOA of H+4 d for secondary fallout was derived from the temporal variation of the results of measurements of daily total beta-concentration in air (Figure 5 ).
).Estimation of the ground deposition density
 ) calculated the deposition densities of radionuclides, normalized to an exposure rate of 1 mR h-1 at 12 hours post-detonation (H+12h), for different types and platforms of nuclear weapons tests and for different times of arrival of fallout (TOA). Hicks (1981
) calculated the deposition densities of radionuclides, normalized to an exposure rate of 1 mR h-1 at 12 hours post-detonation (H+12h), for different types and platforms of nuclear weapons tests and for different times of arrival of fallout (TOA). Hicks (1981 ) data indicate that although the variability of the normalized deposition density for specific radionuclides from test to test may be substantial, there is little difference in the total deposition densities. Using data from 33 representative tests conducted at the NTS, deposition densities were calculated for fractionation values (ratios of refractory and of volatile radionuclides, R/V) equal to 0.5 (15 tests) for tower tests and equal to 1.0 (18 tests) for balloon tests. Use of the mixture of R/V values reflects conditions at French Polynesia where direct fallout occurred in islands close to the test sites (R/V = 1.0) as well as secondary fallout in distant locations with TOA up to H+20 d (R/V = 0.5). Table 7
) data indicate that although the variability of the normalized deposition density for specific radionuclides from test to test may be substantial, there is little difference in the total deposition densities. Using data from 33 representative tests conducted at the NTS, deposition densities were calculated for fractionation values (ratios of refractory and of volatile radionuclides, R/V) equal to 0.5 (15 tests) for tower tests and equal to 1.0 (18 tests) for balloon tests. Use of the mixture of R/V values reflects conditions at French Polynesia where direct fallout occurred in islands close to the test sites (R/V = 1.0) as well as secondary fallout in distant locations with TOA up to H+20 d (R/V = 0.5). Table 7 shows the medians of the normalized total deposition densities and deposition densities of important radionuclides at different TOAs derived from reports of Hicks (1981
shows the medians of the normalized total deposition densities and deposition densities of important radionuclides at different TOAs derived from reports of Hicks (1981 ). These tabulated values were used to reconstruct fallout from tests conducted in French Polynesia.
). These tabulated values were used to reconstruct fallout from tests conducted in French Polynesia.
Tableau 7 Calculated median deposition densities of selected radionuclides at different TOAs normalized to an exposure rate of 1 mR h-1 at 12 hours post-detonation (H+12h) (estimated from Hicks, 1981 )
)
|
Radio-nuclide
|
Half-lifea
|
Normalized deposition density (Bq m-2 per mR h-1 at H+12h) at TOA
|
|||||||
|
H+6h
|
H+9h
|
H+12h
|
H+1d
|
H+2d
|
H+5d
|
H+10d
|
H+20d
|
||
|
54Mn
|
312.3 d
|
1.4
|
1.4
|
1.4
|
1.4
|
1.4
|
1.4
|
1.3
|
1.3
|
|
89Sr
|
50.53 d
|
4.7 × 103
|
4.7 × 103
|
4.7 × 103
|
3.7 × 103
|
3.7 × 103
|
3.5 × 103
|
3.3 × 103
|
2.9 × 103
|
|
90Sr
|
28.79 y
|
25
|
25
|
25
|
25
|
25
|
25
|
25
|
25
|
|
90Y
|
64.1 h
|
–
|
–
|
–
|
5.8
|
10
|
18
|
24
|
25
|
|
91Sr
|
9.63 h
|
4.1 × 105
|
3.3 × 105
|
2.7 × 105
|
1.1 × 105
|
2.0 × 104
|
1.2 × 102
|
–
|
–
|
|
91mY
|
49.71 m
|
2.7 × 105
|
2.2 × 105
|
1.8 × 105
|
7.4 × 104
|
1.3 × 104
|
76
|
–
|
–
|
|
91Y
|
58.51 d
|
1.4 × 103
|
1.9 × 103
|
2.4 × 103
|
3.5 × 103
|
4.1 × 103
|
4.1 × 103
|
3.9 × 103
|
3.5 × 103
|
|
93Y
|
10.18 h
|
3.6 × 105
|
2.9 × 105
|
2.4 × 105
|
1.0 × 105
|
2.0 × 104
|
1.5 × 102
|
–
|
-
|
|
95Zr
|
64.03 d
|
4.4 × 103
|
4.4 × 103
|
4.4 × 103
|
4.1 × 103
|
4.1 × 103
|
4.0 × 103
|
3.7 × 103
|
3.4 × 103
|
|
95Nb
|
34.99 d
|
20
|
31
|
41
|
80
|
1.6 × 102
|
3.8 × 102
|
7.0 × 102
|
1.2 × 103
|
|
97Zr
|
16.744 h
|
3.0 × 105
|
2.7 × 105
|
2.4 × 105
|
1.5 × 105
|
5.5 × 104
|
2.9 × 103
|
22
|
–
|
|
97mNb
|
60 s b
|
2.9 × 105
|
2.6 × 105
|
2.3 × 105
|
1.4 × 105
|
5.3 × 104
|
2.8 × 103
|
21
|
–
|
|
97Nb
|
72.1 m
|
3.1 × 105
|
2.8 × 105
|
2.5 × 105
|
1.5 × 105
|
5.5 × 104
|
2.9 × 103
|
22
|
–
|
|
99Mo
|
65.94 h
|
9.7 × 104
|
9.4 × 104
|
9.1 × 104
|
8.1 × 104
|
6.3 × 104
|
3.0 × 104
|
8.7 × 103
|
7.0 × 102
|
|
99mTc
|
6.015 h
|
4.4 × 104
|
5.5 × 104
|
6.2 × 104
|
7.1 × 104
|
6.0 × 104
|
2.9 × 104
|
8.3 × 103
|
6.7 × 102
|
|
103Ru
|
39.26 d
|
6.9 × 103
|
6.9 × 103
|
6.9 × 103
|
6.8 × 103
|
6.6 × 103
|
6.4 × 103
|
5.8 × 103
|
4.9 × 103
|
|
106Ru
|
373.59 d
|
3.7 × 102
|
3.7 × 102
|
3.7 × 102
|
3.7 × 102
|
3.7 × 102
|
3.7 × 102
|
3.6 × 102
|
3.6 × 102
|
|
125Sb
|
2.76 y
|
4.7
|
4.7
|
4.8
|
5.0
|
5.5
|
6.7
|
8.3
|
10
|
|
131I
|
8.02 d
|
2.9 × 104
|
2.9 × 104
|
2.8 × 104
|
2.7 × 104
|
2.5 × 104
|
2.0 × 104
|
1.3 × 104
|
5.6 × 103
|
|
132Te
|
3.204 d
|
8.2 × 104
|
8.0 × 104
|
7.8 × 104
|
7.0 × 104
|
5.7 × 104
|
3.0 × 104
|
1.0 × 104
|
1.2 × 103
|
|
132I
|
2.30 h
|
8.5 × 104
|
8.2 × 104
|
8.0 × 104
|
7.2 × 104
|
5.8 × 104
|
3.1 × 104
|
1.1 × 104
|
1.3 × 103
|
|
133I
|
20.8 h
|
3.9 × 105
|
3.6 × 105
|
3.2 × 105
|
2.0 × 104
|
9.1 × 104
|
8.4 × 103
|
1.6 × 102
|
–
|
|
135I
|
6.57 h
|
6.4 × 105
|
4.7 × 105
|
3.4 × 105
|
1.0 × 105
|
8.3 × 103
|
4.8
|
–
|
–
|
|
136Cs
|
13.16 d
|
2.8 × 102
|
2.8 × 102
|
2.8 × 102
|
2.7 × 102
|
2.5 × 102
|
2.2 × 102
|
1.7 × 102
|
98
|
|
137Cs
|
30.17 y
|
34
|
34
|
34
|
34
|
34
|
34
|
34
|
34
|
|
140Ba
|
12.75 d
|
2.4 × 104
|
2.4 × 104
|
2.4 × 104
|
2.3 × 104
|
2.2 × 104
|
1.8 × 104
|
1.4 × 104
|
8.1 × 103
|
|
140La
|
1.68 d
|
2.4 × 103
|
3.4 × 103
|
4.4 × 103
|
7.9 × 103
|
1.3 × 104
|
1.8 × 104
|
1.6 × 104
|
9.4 × 103
|
|
141Ce
|
32.51 d
|
5.2 × 103
|
6.5 × 103
|
7.2 × 103
|
8.6 × 103
|
8.5 × 103
|
8.0 × 103
|
7.2 × 103
|
5.8 × 103
|
|
143Ce
|
30.039 h
|
1.6 × 105
|
1.5 × 105
|
1.4 × 105
|
1.1 × 105
|
6.6 × 104
|
1.4 × 104
|
1.2 × 103
|
7.5
|
|
143Pr
|
13.57 d
|
2.0 × 103
|
3.0 × 103
|
3.9 × 103
|
7.0 × 103
|
1.1 × 104
|
1.4 × 104
|
1.2 × 104
|
7.3 × 103
|
|
144Ce
|
284.91 d
|
7.1 × 102
|
7.0 × 102
|
7.0 × 102
|
7.0 × 102
|
7.0 × 102
|
7.0 × 102
|
6.9 × 102
|
6.7 × 102
|
|
147Nd
|
10.98 d
|
1.0 × 104
|
9.9 × 103
|
9.8 × 103
|
8.8 × 103
|
8.3 × 103
|
7.2 × 103
|
5.0 × 103
|
2.7 × 103
|
|
239Np
|
2.357 d
|
5.0 × 105
|
4.8 × 105
|
4.6 × 105
|
4.0 × 105
|
3.0 × 105
|
1.3 × 105
|
2.8 × 104
|
1.5 × 103
|
|
Total
|
5.0 × 106
|
3.6 × 106
|
3.1 × 106
|
1.9 × 106
|
1.0 × 106
|
3.8 × 105
|
1.5 × 105
|
6.1 × 104
|
|
|
Exposure rate (mR h-1)
|
2.3
|
1.4
|
1.0
|
0.44
|
0.19
|
0.063
|
0.027
|
0.012
| |
a ICRP (2008 ).
).
b (Eckerman and Ryman, 1993 ).
).
 ) to the measured total deposition. If measurement of total deposition on the ground surface was not available, the following approaches were used to determine the deposition densities of the various radionuclides, depending on the type of data available for the locations of interest.
) to the measured total deposition. If measurement of total deposition on the ground surface was not available, the following approaches were used to determine the deposition densities of the various radionuclides, depending on the type of data available for the locations of interest.• Approach #1. An exposure-rate measurement was available
 ).
). ).
).• Approach #2. Measurements of 131I concentration in milk
were available:
 ) to that obtained in step 1 as a scale.
) to that obtained in step 1 as a scale.• Approach #3. A measurement of total beta-activity in filtered air
was available:
 ) to that obtained in step 1.
) to that obtained in step 1. ) for detail).
) for detail). shows the total and the 131I deposition densities estimated for these 49 islands and atolls and indicate the test that contributed the most to the fallout that occurred in each location. The tests that contributed the most to the radioactive fallout in each archipelago of French Polynesia were:
shows the total and the 131I deposition densities estimated for these 49 islands and atolls and indicate the test that contributed the most to the fallout that occurred in each location. The tests that contributed the most to the radioactive fallout in each archipelago of French Polynesia were: gives examples of radiation data available for Tahiti and of reconstructed deposition densities. As mentioned above, test Centaure (17/07/1974) resulted in the highest radioactive contamination of the most populated island in French Polynesia. Tests Sirius (4/10/1966) and Arcturus (2/07/1967) also resulted in substantial deposition in Tahiti. All other tests contributed less than 6% to the total deposition from all tests. Regarding 131I, tests Centaure, Sirius and Arcturus contributed around 85% of the 131I deposition in Tahiti.
gives examples of radiation data available for Tahiti and of reconstructed deposition densities. As mentioned above, test Centaure (17/07/1974) resulted in the highest radioactive contamination of the most populated island in French Polynesia. Tests Sirius (4/10/1966) and Arcturus (2/07/1967) also resulted in substantial deposition in Tahiti. All other tests contributed less than 6% to the total deposition from all tests. Regarding 131I, tests Centaure, Sirius and Arcturus contributed around 85% of the 131I deposition in Tahiti. ) (Table 10
) (Table 10 ). The ratios of the deposition densities estimated in this study by different approaches to the deposition densities reported by SMSR and Bourges (1997
). The ratios of the deposition densities estimated in this study by different approaches to the deposition densities reported by SMSR and Bourges (1997 ) are characterized by an arithmetic mean ± standard deviation of 0.9±0.4, a geometric mean of 0.8 and range from 0.2 to 1.5 for approach #1 (13 deposition events); the corresponding values for approach #2 (8 deposition events) are 1.2±1.2 for the arithmetic mean, 0.9 for the geometric mean, and 0.4-4.0 for the range; for approach #3 (3 deposition events), the obtained values are 0.6±0.4 for the arithmetic mean, 0.6 for the geometric mean, and 0.4-1.1 for the range. For most deposition events (19 from 24, 79.2% of the total) a good agreement (within a factor of 2) was observed between the deposition densities estimated in this study and those reported in the literature.
) are characterized by an arithmetic mean ± standard deviation of 0.9±0.4, a geometric mean of 0.8 and range from 0.2 to 1.5 for approach #1 (13 deposition events); the corresponding values for approach #2 (8 deposition events) are 1.2±1.2 for the arithmetic mean, 0.9 for the geometric mean, and 0.4-4.0 for the range; for approach #3 (3 deposition events), the obtained values are 0.6±0.4 for the arithmetic mean, 0.6 for the geometric mean, and 0.4-1.1 for the range. For most deposition events (19 from 24, 79.2% of the total) a good agreement (within a factor of 2) was observed between the deposition densities estimated in this study and those reported in the literature. ).
).
Tableau 8 Total and 131I deposition densities from atmospheric nuclear weapons tests conducted in French Polynesia for the 49 islands and atolls where the study subjects resided in 1966-1974 (Drozdovitch et al., 2020a )
)
|
Archipelago
|
Island
|
Deposition density from all tests (Bq m-2)
|
Most important contributor
|
Date of test (dd/mm/yyyy)
|
TOA
|
Deposition density from the most important test (Bq m-2)
|
||
|
Total
|
131I
|
Total
|
131I
|
|||||
|
Society
|
Tahiti
|
4.3 × 106
|
1.3 × 105
|
Centaure
|
17/07/1974
|
H+56h
|
3.4 × 106
|
9.5 × 104
|
|
Bora-Bora
|
1.1 × 106
|
4.4 × 104
|
Centaure
|
17/07/1974
|
H+2.5d
|
8.8 × 105
|
2.6 × 104
|
|
|
Huahine
|
1.1 × 106
|
4.3 × 104
|
Centaure
|
17/07/1974
|
H+2.5d
|
7.5 × 105
|
2.2 × 104
|
|
|
Maiao
|
3.8 × 106
|
1.2 × 105
|
Centaure
|
17/07/1974
|
H+2.5d
|
3.3 × 106
|
1.0 × 105
|
|
|
Maupiti
|
1.1 × 106
|
4.4 × 104
|
Centaure
|
17/07/1974
|
H+2.5d
|
8.8 × 105
|
2.6 × 104
|
|
|
Moorea
|
1.2 × 106
|
4.4 × 104
|
Centaure
|
17/07/1974
|
H+58h
|
1.0 × 106
|
3.0 × 104
|
|
|
Raiatea
|
1.1 × 106
|
4.1 × 104
|
Centaure
|
17/07/1974
|
H+2.5d
|
7.5 × 105
|
2.2 × 104
|
|
|
Tahaa
|
1.1 × 106
|
4.1 × 104
|
Centaure
|
17/07/1974
|
H+2.5d
|
7.5 × 105
|
2.2 × 104
|
|
|
Tuamotu-Gambier
|
Ahe
|
4.9 × 105
|
3.3 × 104
|
Sirius
|
04/10/1966
|
H+5d
|
1.3 × 105
|
6.9 × 103
|
|
Anaa
|
5.9 × 106
|
1.7 × 105
|
Centaure
|
17/07/1974
|
H+2d
|
4.1 × 106
|
1.0 × 105
|
|
|
Apataki
|
5.7 × 105
|
3.7 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
1.3 × 105
|
8.1 × 103
|
|
|
Arutua
|
5.7 × 105
|
3.7 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
1.3 × 105
|
8.1 × 103
|
|
|
Faaite
|
1.4 × 106
|
7.3 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
5.8 × 105
|
2.0 × 104
|
|
|
Fakarava
|
1.2 × 106
|
6.8 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
4.6 × 105
|
1.6 × 104
|
|
|
Fangatau
|
6.2 × 105
|
4.1 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
1.5 × 105
|
5.2 × 103
|
|
|
Gambier
|
7.2 × 107
|
7.6 × 105
|
Aldébaran a
|
02/07/1966
|
H+10h45/ H+13d
|
6.1 × 107/
1.6 × 104
|
5.4 × 105/
1.5 × 103
|
|
|
Hao
|
1.4 × 106
|
3.9 × 104
|
Arcturus
|
02/07/1967
|
H+33h
|
9.2 × 105
|
1.6 × 104
|
|
|
Katiu
|
1.7 × 106
|
8.9 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
6.7 × 105
|
2.3 × 104
|
|
|
Kauehi
|
1.5 × 106
|
7.8 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
5.7 × 105
|
2.0 × 104
|
|
|
Kaukura
|
5.8 × 105
|
3.8 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
1.3 × 105
|
8.1 × 103
|
|
|
Makatea
|
1.7 × 106
|
5.5 × 104
|
Centaure
|
17/07/1974
|
H+3d
|
1.4 × 106
|
3.4 × 104
|
|
|
Makemo
|
1.8 × 106
|
9.6 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
6.7 × 105
|
2.3 × 104
|
|
|
Manihi
|
4.9 × 105
|
3.3 × 104
|
Sirius
|
04/10/1966
|
H+5d
|
1.3 × 105
|
6.9 × 103
|
|
|
Marokau
|
1.1 × 106
|
3.5 × 104
|
Arcturus
|
02/07/1967
|
H+36h
|
7.3 × 105
|
1.4 × 104
|
|
|
Mataiva
|
2.5 × 105
|
1.8 × 104
|
Altaïr
|
05/06/1967
|
H+11d
|
3.5 × 104
|
3.3 × 103
|
|
|
Napuka
|
6.2 × 105
|
4.1 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
1.4 × 105
|
5.2 × 103
|
|
|
Niau
|
1.2 × 106
|
6.8 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
4.6 × 105
|
1.6 × 104
|
|
|
Nukutavake
|
5.3 × 105
|
3.2 × 104
|
Sirius
|
04/10/1966
|
H+5d
|
1.2 × 105
|
6.2 × 103
|
|
|
Pukarua
|
1.2 × 107
|
2.5 × 105
|
Arcturus
|
02/07/1967
|
H+38h
|
1.1 × 107
|
2.1 × 105
|
|
|
Rangiroa
|
2.4 × 105
|
1.7 × 104
|
Altaïr
|
05/06/1967
|
H+11d
|
3.5 × 104
|
3.3 × 103
|
|
|
Raroia
|
6.2 × 105
|
4.1 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
1.5 × 105
|
5.2 × 103
|
|
|
Reao
|
1.2 × 107
|
2.6 × 105
|
Arcturus
|
02/07/1967
|
H+36h
|
1.1 × 107
|
2.1 × 105
|
|
|
Taenga
|
1.8 × 106
|
9.6 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
6.7 × 105
|
2.3 × 104
|
|
|
Takapoto
|
4.9 × 105
|
3.3 × 104
|
Sirius
|
04/10/1966
|
H+5d
|
1.3 × 105
|
6.9 × 103
|
|
|
Takume
|
6.2 × 105
|
4.1 × 104
|
Arcturus
|
02/07/1967
|
H+3d
|
1.5 × 105
|
5.2 × 103
|
|
|
Tatakoto
|
1.2 × 107
|
2.5 × 105
|
Arcturus
|
02/07/1967
|
H+40h
|
1.1 × 107
|
2.1 × 105
|
|
|
Tikehau
|
2.5 × 105
|
1.8 × 104
|
Altaïr
|
05/06/1967
|
H+11d
|
3.6 × 104
|
3.3 × 103
|
|
|
Tureia
|
4.0 × 107
|
3.9 × 105
|
Arcturus
|
02/07/1967
|
H+11h40
|
1.6 × 107
|
1.5 × 105
|
|
|
Marquesas
|
Fatu Hiva
|
2.9 × 105
|
2.1 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
8.2 × 104
|
5.0 × 103
|
|
Hiva Oa
|
5.2 × 105
|
3.5 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
2.9 × 105
|
1.8 × 104
|
|
|
Nuku Hiva
|
1.9 × 105
|
1.3 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
8.2 × 104
|
5.0 × 103
|
|
|
Tahuata
|
5.2 × 105
|
3.5 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
2.9 × 105
|
1.8 × 104
|
|
|
Ua Huka
|
2.0 × 105
|
1.4 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
8.2 × 104
|
5.0 × 103
|
|
|
Ua Pou
|
2.0 × 105
|
1.3 × 104
|
Sirius
|
04/10/1966
|
H+6d
|
8.2 × 104
|
5.0 × 103
|
|
|
Austral
|
Raivavae
|
5.1 × 105
|
1.9 × 104
|
Pallas
|
18/08/1973
|
H+3d
|
3.8 × 105
|
1.3 × 104
|
|
Rapa
|
4.1 × 105
|
2.2 × 104
|
Pallas
|
18/08/1973
|
H+5d
|
3.2 × 105
|
1.7 × 104
|
|
|
Rimatara
|
5.1 × 105
|
1.9 × 104
|
Pallas
|
18/08/1973
|
H+3d
|
3.8 × 105
|
1.3 × 104
|
|
|
Rurutu
|
5.1 × 105
|
1.9 × 104
|
Pallas
|
18/08/1973
|
H+3d
|
3.8 × 105
|
1.3 × 104
|
|
|
Tubuai
|
5.2 × 105
|
1.9 × 104
|
Pallas
|
18/08/1973
|
H+3d
|
3.8 × 105
|
1.3 × 104
|
|
a Direct fallout / secondary fallout.
Tableau 9 Reconstructed deposition densities on Tahiti island from the atmospheric nuclear weapons tests conducted in French Polynesia (Drozdovitch et al., 2020a )
)
|
Name of test
|
Date of test
|
TOA
|
Time-integrated beta-concentration in air
(Bq s m-3)
|
Precipi-
tation
(mm)
|
Deposition density
(Bq m-2)
|
|
|
Total
|
131I
|
|||||
|
Aldébaran
|
02/07/1966
|
H+10d
|
2.7 × 105
|
0
|
4.7 × 103
|
4.1 × 102
|
|
Tamouré
|
19/07/1966
|
H+8d
|
1.1 × 105
|
308
|
7.0 × 103
|
5.2 × 102
|
|
Bételgeuse
|
11/09/1966
|
H+10d
|
4.1 × 105
|
0
|
7.1 × 103
|
6.2 × 102
|
|
Rigel
|
24/09/1966
|
H+4d
|
7.0 × 104
|
0
|
1.2 × 103
|
54
|
|
Sirius a
|
04/10/1966
|
H+18h / H+7d
|
6.4 × 106 / 2.3 × 106
|
151 / 0
|
4.0 × 105 / 4.0 × 104
|
4.5 × 103 /
2.7 × 103
|
|
Altaïr
|
05/06/1967
|
H+13d
|
3.8 × 105
|
0
|
6.7 × 103
|
6.5 × 102
|
|
Antarès
|
27/06/1967
|
H+4d
|
2.7 × 105
|
0
|
4.7 × 103
|
2.1 × 102
|
|
Arcturus
|
02/07/1967
|
H+4d
|
6.4 × 106
|
0
|
1.1 × 105
|
4.9 × 103
|
|
Capella
|
07/07/1968
|
H+7d
|
2.4 × 105
|
0
|
4.1 × 103
|
2.8 × 102
|
|
Castor
|
15/07/1968
|
H+14d
|
1.8 × 105
|
0
|
3.2 × 103
|
3.1 × 102
|
|
Pollux
|
03/08/1968
|
H+12d
|
6.3 × 105
|
0
|
1.1 × 104
|
1.1 × 103
|
|
Canopus
|
24/08/1968
|
H+6d
|
2.2 × 105
|
86
|
1.4 × 104
|
8.3 × 102
|
|
Procyon
|
08/09/1968
|
H+20d
|
1.2 × 105
|
0
|
2.1 × 103
|
2.0 × 102
|
|
Andromède
|
15/05/1970
|
H+16d
|
7.1 × 104
|
0
|
1.2 × 103
|
1.2 × 102
|
|
Cassiopée
|
22/05/1970
|
H+12d
|
4.5 × 104
|
0
|
7.9 × 102
|
75
|
|
Dragon
|
30/05/1970
|
H+4d
|
1.6 × 104
|
0
|
2.7 × 102
|
12
|
|
Eridan
|
24/06/1970
|
H+15d
|
2.3 × 104
|
0
|
4.0 × 102
|
39
|
|
Licorne
|
03/07/1970
|
H+10d
|
1.5 × 105
|
8
|
9.6 × 103
|
8.4 × 102
|
|
Pégase
|
27/07/1970
|
H+10d
|
1.6 × 105
|
3
|
9.9 × 103
|
8.7 × 102
|
|
Orion
|
02/08/1970
|
H+14d
|
1.9 × 105
|
1
|
1.2 × 104
|
1.1 × 103
|
|
Toucan
|
06/08/1970
|
H+4d
|
4.1 × 105
|
1
|
2.6 × 104
|
1.1 × 103
|
|
Dioné
|
05/06/1971
|
H+10d
|
5.9 × 104
|
2
|
3.7 × 103
|
3.2 × 102
|
|
Encelade
|
12/06/1971
|
H+11d
|
1.4 × 106
|
0
|
2.3 × 104
|
2.1 × 103
|
|
Japet
|
04/07/1971
|
H+8d
|
2.1 × 105
|
0
|
3.7 × 103
|
2.8 × 102
|
|
Phoebé
|
08/08/1971
|
H+6d
|
4.0 × 104
|
0
|
7.0 × 102
|
43
|
|
Rhéa
|
14/08/1971
|
H+15d
|
9.5 × 104
|
2
|
5.9 × 103
|
5.8 × 102
|
|
Umbriel b
|
25/06/1972
|
H+2d
|
–
|
0
|
4.7 × 103
|
1.2 × 102
|
|
Titania
|
30/06/1972
|
H+10d
|
5.9 × 104
|
0
|
1.0 × 103
|
91
|
|
Obéron
|
27/07/1972
|
H+17d
|
1.0 × 105
|
45
|
6.5 × 103
|
6.3 × 102
|
|
Euterpe
|
21/07/1973
|
H+21d
|
1.4 × 104
|
16
|
8.5 × 102
|
76
|
|
Melpomène
|
28/07/1973
|
–
|
–
|
–
|
–
|
–
|
|
Pallas c
|
18/08/1973
|
H+3d
|
–
|
5
|
4.7 × 104
|
1.6 × 103
|
|
Parthénope c
|
24/08/1973
|
H+7d
|
–
|
31
|
1.2 × 104
|
8.3 × 102
|
|
Tamara
|
28/08/1973
|
H+2d
|
–
|
2
|
1.3 × 104
|
3.1 × 102
|
|
Capricorne
|
16/06/1974
|
H+5d
|
1.0 × 105
|
4
|
6.3 × 103
|
3.4 × 102
|
|
Gémeaux
|
07/07/1974
|
H+8d
|
2.0 × 104
|
0
|
3.5 × 102
|
22
|
|
Centaure
|
17/07/1974
|
H+56h
|
5.5 × 107
|
1
|
3.4 × 106
|
9.5 × 104
|
|
Maquis
|
25/07/1974
|
H+5d
|
1.0 × 105
|
0
|
1.8 × 103
|
96
|
|
Scorpion
|
15/08/1974
|
H+2d
|
2.9 × 104
|
0
|
5.2 × 102
|
13
|
|
Taureau
|
24/08/1974
|
H+6d
|
4.7 × 104
|
0
|
8.3 × 102
|
50
|
|
Verseau
|
14/10/1974
|
H+6d
|
7.0 × 104
|
0
|
1.2 × 103
|
75
|
a Direct / secondary fallout.
b Using approach #3.
c Reconstructed using measured total deposition.
Tableau 10 Deposition densities at TOA reconstructed in this study using different approaches and measured (Bourges, 1997 ; SMSR, 1966b
; SMSR, 1966b , 1967b
, 1967b , 1968
, 1968 -1969
-1969 , 1970c
, 1970c , 1971a
, 1971a , 1973b
, 1973b , 1974a
, 1974a , b
, b , 1975
, 1975 )
)
|
Name of test
|
Date of test
|
Archipe-
lago
|
Island (atoll)
|
TOA
|
Deposition density (Bq m-2)
|
|||
|
Reconstructed using approach
|
Measureda
|
|||||||
|
#1
|
#2
|
#3
|
||||||
|
Aldébaran
|
02/07/1966
|
Gambier
|
Gambier
|
H+10h45
|
–
|
6.1 × 107
|
–
|
6.0 × 107 b
|
|
Rigel
|
24/09/1966
|
Tuamotu
|
Tureia
|
H+12h30
|
2.0 × 105
|
–
|
–
|
5.0 × 105
|
|
Arcturus
|
02/07/1967
|
Tuamotu
|
Hao
|
H+33h
|
8.0 × 105
|
–
|
–
|
9.2 × 105
|
|
Arcturus
|
02/07/1967
|
Tuamotu
|
Tureia
|
H+11h40
|
–
|
1.5 × 107
|
–
|
1.6 × 107
|
|
Canopus
|
24/08/1968
|
Tuamotu
|
Reao
|
H+24h
|
5.5 × 103
|
–
|
–
|
8.9 × 103
|
|
Andromède
|
15/05/1970
|
Society
|
Tahiti
|
H+16d
|
1.2 × 103
|
–
|
7.9 × 102
|
–
|
|
Dragon
|
30/05/1970
|
Tuamotu
|
Tureia
|
H+31h
|
–
|
2.1 × 105
|
–
|
2.4 × 105
|
|
Dragon
|
30/05/1970
|
Austral
|
Rapa
|
H+56h
|
2.9 × 103
|
–
|
–
|
3.4 × 103
|
|
Orion
|
02/08/1970
|
Society
|
Tahiti
|
H+14d
|
1.2 × 104
|
–
|
1.4 × 104
|
–
|
|
Dioné
|
05/06/1971
|
Society
|
Tahiti
|
H+10d
|
3.7 × 103
|
–
|
8.9 × 103
|
–
|
|
Encelade
|
12/06/1971
|
Society
|
Tahiti
|
H+11d
|
2.3 × 103
|
–
|
3.4 × 103
|
–
|
|
Encelade
|
12/06/1971
|
Tuamotu
|
Tureia
|
H+12h
|
–
|
1.2 × 107
|
–
|
1.3 × 107 b
|
|
Phoebé
|
08/08/1971
|
Gambier
|
Gambier
|
H+11h15
|
1.0 × 107
|
9.2 × 106
|
–
|
–
|
|
Umbriel
|
25/06/1972
|
Society
|
Tahiti
|
H+2d
|
3.2 × 103
|
–
|
4.7 × 103
|
–
|
|
Pallas
|
18/08/1973
|
Austral
|
Raivavae
|
H+3d
|
3.8 × 105
|
1.6 × 105
|
–
|
–
|
|
Pallas
|
18/08/1973
|
Austral
|
Tubuai
|
H+3d
|
3.8 × 105
|
1.5 × 105
|
–
|
–
|
|
Parthénope
|
24/08/1973
|
Society
|
Tahiti
|
H+7d
|
1.6 × 104
|
–
|
5.8 × 103
|
1.2 × 104
|
|
Tamara
|
28/08/1973
|
Society
|
Tahiti
|
H+2d
|
1.6 × 104
|
–
|
1.4 × 104
|
1.3 × 104
|
|
Tamara
|
28/08/1973
|
Tuamotu
|
Hao
|
H+24h
|
5.8 × 104
|
–
|
–
|
5.3 × 104
|
|
Tamara
|
28/08/1973
|
Tuamotu
|
Reao
|
H+2d
|
6.1 × 103
|
–
|
–
|
1.5 × 104
|
|
Centaurec
|
17/07/1974
|
Society
|
Tahiti
|
H+56h
|
3.4 × 106
|
2.0 × 106
|
1.3 × 106
|
3.4 × 106
|
|
Centaure
|
17/07/1974
|
Tuamotu
|
Tureia
|
H+56h
|
3.9 × 104
|
2.0 × 104
|
–
|
4.5 × 104
|
|
Scorpion
|
15/08/1974
|
Tuamotu
|
Reao
|
H+12h
|
–
|
9.3 × 103
|
–
|
2.3 × 103
|
|
Taureau
|
24/08/1974
|
Tuamotu
|
Gambier
|
H+18h
|
1.3 × 104
|
3.4 × 104
|
–
|
5.7 × 104
|
|
Taureau
|
24/08/1974
|
Tuamotu
|
Reao
|
H+5d
|
2.2 × 104
|
–
|
–
|
1.5 × 104
|
|
Taureau
|
24/08/1974
|
Tuamotu
|
Tureia
|
H+5d
|
4.9 × 103
|
–
|
–
|
5.4 × 103
|
a Declassified report of Joint Radiological Safety Service (SMSR, 1966b , 1967b
, 1967b , 1968
, 1968 -1969
-1969 , 1970c
, 1970c , 1971a
, 1971a , 1973b
, 1973b , 1974a
, 1974a , b
, b , 1975
, 1975 ), unless otherwise indicated.
), unless otherwise indicated.
b Bourges (1997 ).
).
c Measured deposition density is given for Mahina.
Thyroid doses to French Polynesians resulting from atmospheric nuclear weapons tests: estimates based on radiation measurements
and population lifestyle data
 ) and TD19 (Drozdovitch et al., 2020b
) and TD19 (Drozdovitch et al., 2020b ). As it was indicated above, new radiation measurements available from declassified reports and population lifestyle and consumption data collected during the focus group study were used in TD19.
). As it was indicated above, new radiation measurements available from declassified reports and population lifestyle and consumption data collected during the focus group study were used in TD19.Assessment of the internal doses resulting from inhalation
 ); RFair is the reduction factor associated with indoor occupancy (unitless). As buildings in French Polynesia are very open for outdoor air circulation, RFair = 1 was applied in the calculations; TIAiair is the time-integrated concentration of radionuclide i in air (Bq s m-3); DCi,kinh is the age-dependent inhalation dose coefficient for the thyroid, i.e. the thyroid dose due to inhalation of unit activity of radionuclide i by a study subject of age k (mGy Bq-1) (ICRP, 1995
); RFair is the reduction factor associated with indoor occupancy (unitless). As buildings in French Polynesia are very open for outdoor air circulation, RFair = 1 was applied in the calculations; TIAiair is the time-integrated concentration of radionuclide i in air (Bq s m-3); DCi,kinh is the age-dependent inhalation dose coefficient for the thyroid, i.e. the thyroid dose due to inhalation of unit activity of radionuclide i by a study subject of age k (mGy Bq-1) (ICRP, 1995 ).
). ). The value of the time-integrated concentration in air of specific radionuclide i was derived from the radionuclide mix at time of arrival of fallout (TOA) calculated by Hicks (1981
). The value of the time-integrated concentration in air of specific radionuclide i was derived from the radionuclide mix at time of arrival of fallout (TOA) calculated by Hicks (1981 ) assuming that the radionuclide composition in filtered air was the same as that in the activity deposited on the ground. If the total deposition density was measured, an estimate of the time-integrated concentration of radionuclide i in air was obtained from the deposition density and the effective deposition velocity of radionuclide onto the ground surface:
) assuming that the radionuclide composition in filtered air was the same as that in the activity deposited on the ground. If the total deposition density was measured, an estimate of the time-integrated concentration of radionuclide i in air was obtained from the deposition density and the effective deposition velocity of radionuclide onto the ground surface: ) or wet deposition (Drozdovitch et al., 2008
) or wet deposition (Drozdovitch et al., 2008 ), respectively. The assumption was made that the radionuclide distribution in the deposited activity was not influenced by the type of deposition, i.e. wet vs dry.
), respectively. The assumption was made that the radionuclide distribution in the deposited activity was not influenced by the type of deposition, i.e. wet vs dry.Thyroid dose due to ingestion of radioiodine isotopes and 132Te
 , 1996a
, 1996a ); Vm,k is the consumption rate of foodstuff m and drinking water by the subject of age k (kg (L) d-1); PFi,m is the processing factor, i.e., the fraction of radionuclide i remaining in foodstuff m after washing, culinary preparation and time delay between production and consumption (unitless); TIAi,mfood is the time-integrated concentration of radionuclide i in foodstuff or drinking water m (Bq d kg-1 (L-1)).
); Vm,k is the consumption rate of foodstuff m and drinking water by the subject of age k (kg (L) d-1); PFi,m is the processing factor, i.e., the fraction of radionuclide i remaining in foodstuff m after washing, culinary preparation and time delay between production and consumption (unitless); TIAi,mfood is the time-integrated concentration of radionuclide i in foodstuff or drinking water m (Bq d kg-1 (L-1)).• Estimation of consumption rates at age k from the consumption rates reported for age 15
 shows, as example, values of the scaling factor, SFm,k, that were derived for Tahiti and Tuamotu archipelago (except Gambier Islands) from a focus-group survey of dietary patterns in French Polynesia (Drozdovitch et al., 2020b
shows, as example, values of the scaling factor, SFm,k, that were derived for Tahiti and Tuamotu archipelago (except Gambier Islands) from a focus-group survey of dietary patterns in French Polynesia (Drozdovitch et al., 2020b ).
).
Tableau 11 Scaling factors, SFm,k, for age-dependent consumption rates of foodstuffs for Tahiti and Tuamotu archipelago (except Gambier Islands) (Drozdovitch et al., 2020b )
)
|
Foodstuff
|
Tahiti
|
Tuamotu archipelago (except Gambier Islands)
|
||||||||
|
0-12 mo
|
1-3.9 y
|
4-6.9 y
|
7-14.9 y
|
15-21 y
|
0-12 mo
|
1-3.9 y
|
4-6.9 y
|
7-14.9 y
|
15-21 y
|
|
|
Fresh cow’s milk
|
-a
|
0.34
|
0.39
|
1.03
|
1.00
|
-
|
-
|
-
|
-
|
-
|
|
Leafy vegetablesb
|
-a
|
0.05
|
0.22
|
0.77
|
1.00
|
-a
|
-a
|
0.14
|
1.20
|
1.00
|
|
Fâfâc
|
-a
|
0.05
|
0.15
|
0.56
|
1.00
|
-
|
-
|
-
|
-
|
-
|
|
Coco milk
|
0.02
|
0.22
|
0.53
|
0.50
|
1.00
|
0.27
|
0.33
|
0.57
|
0.86
|
1.00
|
|
Coco copra
|
0.03
|
0.13
|
0.82
|
0.80
|
1.00
|
-a
|
0.21
|
1.00
|
1.00
|
1.00
|
|
Uru
|
-a
|
0.03
|
0.32
|
0.59
|
1.00
|
-a
|
0.03
|
0.23
|
0.42
|
1.00
|
|
Banana
|
0.03
|
0.32
|
0.64
|
0.77
|
1.00
|
-a
|
0.06
|
0.52
|
0.97
|
1.00
|
|
Mango
|
0.06
|
0.38
|
0.52
|
0.79
|
1.00
|
-d
|
-d
|
-d
|
-d
|
-d
|
|
Papaya
|
0.25
|
0.38
|
0.42
|
0.43
|
1.00
|
-a
|
0.25
|
0.35
|
0.67
|
1.00
|
|
Manioc
|
-a
|
0.26
|
0.53
|
0.68
|
1.00
|
-a
|
-d
|
-d
|
-d
|
-d
|
|
Taro
|
-a
|
0.26
|
0.67
|
0.76
|
1.00
|
-a
|
-d
|
-d
|
-d
|
-d
|
|
Sweet potatoes
|
-a
|
0.31
|
0.70
|
0.81
|
1.00
|
-a
|
-d
|
-d
|
-d
|
-d
|
|
Poultry
|
-a
|
0.14
|
0.63
|
0.74
|
1.00
|
-a
|
0.08
|
0.39
|
1.13
|
1.00
|
|
Beef
|
-a
|
0.13
|
0.81
|
0.96
|
1.00
|
-a
|
-d
|
-d
|
-d
|
-d
|
|
Pork
|
-a
|
0.00
|
0.67
|
0.90
|
1.00
|
-a
|
-a
|
0.23
|
0.46
|
1.00
|
|
Benitier
|
-a
|
0.04
|
0.12
|
0.58
|
1.00
|
-a
|
-a
|
0.22
|
0.41
|
1.00
|
|
Fishe
|
0.01
|
0.19
|
0.39
|
0.73
|
1.00
|
-a
|
0.06
|
0.27
|
0.63
|
1.00
|
a Did not consume this foodstuff at this age.
b Including pota, watercress, spinach, lettuce.
c Leaves of taro.
d Did consume this foodstuff, but it was not locally produced.
e Either from sea or from lagoon.
• Estimation of the time-integrated concentration in local cow’s milk
in Tahiti
 ; NCI, 1997
; NCI, 1997 ):
): ); Ig = 40 kg d-1 is the daily intake of grass by cows, fresh weight; TFi is the cow’s intake-to-milk transfer coefficient of radionuclide i (d L-1). It was taken to be 3×10-3 and 5×10-4 d L-1 for stable iodine and tellurium, respectively (Müller and Pröhl, 1993
); Ig = 40 kg d-1 is the daily intake of grass by cows, fresh weight; TFi is the cow’s intake-to-milk transfer coefficient of radionuclide i (d L-1). It was taken to be 3×10-3 and 5×10-4 d L-1 for stable iodine and tellurium, respectively (Müller and Pröhl, 1993 ); λmi is biological transfer rate in cow’s milk: 0.99 and 0.69 d-1 for stable iodine and tellurium, respectively (Müller and Pröhl, 1993
); λmi is biological transfer rate in cow’s milk: 0.99 and 0.69 d-1 for stable iodine and tellurium, respectively (Müller and Pröhl, 1993 ); λwi is the elimination rate of radionuclide i from grass due to processes of weathering and growth dilution: 0.069 and 0.047 d-1 for stable iodine and tellurium, respectively (Miller and Hoffman, 1983
); λwi is the elimination rate of radionuclide i from grass due to processes of weathering and growth dilution: 0.069 and 0.047 d-1 for stable iodine and tellurium, respectively (Miller and Hoffman, 1983 ; Müller and Pröhl, 1993
; Müller and Pröhl, 1993 ); λri is the radioactive decay constant of radionuclide i (d-1).
); λri is the radioactive decay constant of radionuclide i (d-1). ) provided the opportunity to validate the 131I concentration obtained using this method. The measured (Republic of France, 1975
) provided the opportunity to validate the 131I concentration obtained using this method. The measured (Republic of France, 1975 ) and the calculated 131I concentration in cow’s milk are compared in Figure 6
) and the calculated 131I concentration in cow’s milk are compared in Figure 6 . There is good agreement between the two sets of values.
. There is good agreement between the two sets of values.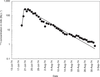 | Figure 6 131I activity in cow’s milk produced in Tahiti after the test Centaure: calculation (curve) and measurements (open circles) (Drozdovitch et al., 2008 ) ) |
• Estimation of the time-integrated concentration in leafy vegetables
 ). For 132Te, the values of the processing factors were taken to be equal to 1.0 for watercress (washing) and 0.9 for fâfâ, pota and spinach (washing and boiling).
). For 132Te, the values of the processing factors were taken to be equal to 1.0 for watercress (washing) and 0.9 for fâfâ, pota and spinach (washing and boiling). , 1969
, 1969 , 1971
, 1971 -1975
-1975 ). Figure 7
). Figure 7 compares the total gamma concentrations in leafy vegetables measured in Tahiti after test Centaure and calculated using this method. In general, the model provides estimates that are in reasonable agreement with the measurements.
compares the total gamma concentrations in leafy vegetables measured in Tahiti after test Centaure and calculated using this method. In general, the model provides estimates that are in reasonable agreement with the measurements.• Estimation of the time-integrated concentration in drinking water
 ) for direct fallout with TOA of H+24h or less. For TOA greater than one day, the solubility of radioiodine isotopes was taken to be equal to 1.
) for direct fallout with TOA of H+24h or less. For TOA greater than one day, the solubility of radioiodine isotopes was taken to be equal to 1. ) (mm); Wcistern is the volume of the cistern equals to 15,000 and 90,000 L for family and communal cisterns, respectively (HCRFP, 1977
) (mm); Wcistern is the volume of the cistern equals to 15,000 and 90,000 L for family and communal cisterns, respectively (HCRFP, 1977 ).
). ). The amount of water in the cisterns was calculated for each year of atmospheric testing for the time period starting 2 weeks before the first nuclear weapons test and ending 5 weeks after the last test of the year. The initial content of rainwater in the cistern was assumed to be 1/4 of its volume. The model considers that the daily consumption of drinking water varied from 0.25 L for 0-12 mo to 2.0 L for adults.
). The amount of water in the cisterns was calculated for each year of atmospheric testing for the time period starting 2 weeks before the first nuclear weapons test and ending 5 weeks after the last test of the year. The initial content of rainwater in the cistern was assumed to be 1/4 of its volume. The model considers that the daily consumption of drinking water varied from 0.25 L for 0-12 mo to 2.0 L for adults.Thyroid dose due to external irradiation from radionuclides deposited
on the ground
 ); t1 and t2 are the times of beginning and end of residence at a given location (d).
); t1 and t2 are the times of beginning and end of residence at a given location (d). ); Tindoors,k is the time spent indoors in a day by a subject of age k, which depends for schoolchildren aged 7-14.9 y on the time of year: school days or school vacation (from 15 June to 15 August) (h). Age-, archipelago- and season-specific values of time spent indoors for the Polynesian population was collected by Drozdovitch et al. (2019
); Tindoors,k is the time spent indoors in a day by a subject of age k, which depends for schoolchildren aged 7-14.9 y on the time of year: school days or school vacation (from 15 June to 15 August) (h). Age-, archipelago- and season-specific values of time spent indoors for the Polynesian population was collected by Drozdovitch et al. (2019 ); LF is the location factor for outdoor conditions that depends on the type of environment (unitless). It was taken to be 0.5 for a typical urban environment and 0.7 for a rural environment (Drozdovitch et al., 2008
); LF is the location factor for outdoor conditions that depends on the type of environment (unitless). It was taken to be 0.5 for a typical urban environment and 0.7 for a rural environment (Drozdovitch et al., 2008 ).
). provides the values for BFk, calculated using eqn (11), that were used in this study. If the study subject reported during the personal interview the type and construction material of residence, the value of the behavioral factor corresponding to age k of the study subject and to her/his archipelago and type of residence was used in the dose calculation. If the study subject did not recall the type and construction material for her/his residence, the behavioral factor was estimated using the archipelago-specific distribution of types and construction materials of residences that were reported by the focus groups to be typical in the 1960s-1970s (Drozdovitch et al., 2019
provides the values for BFk, calculated using eqn (11), that were used in this study. If the study subject reported during the personal interview the type and construction material of residence, the value of the behavioral factor corresponding to age k of the study subject and to her/his archipelago and type of residence was used in the dose calculation. If the study subject did not recall the type and construction material for her/his residence, the behavioral factor was estimated using the archipelago-specific distribution of types and construction materials of residences that were reported by the focus groups to be typical in the 1960s-1970s (Drozdovitch et al., 2019 ).
).Ingestion of long-lived 137Cs with foodstuffs
 ) (mGy Bq-1); Vm,k is the consumption rate of foodstuff m by the subject of age k (see Table 2
) (mGy Bq-1); Vm,k is the consumption rate of foodstuff m by the subject of age k (see Table 2 for the list of foodstuffs) (kg (L) d-1); PFmCs is the processing factor that reflects the change in activity concentration of 137Cs resulting from the culinary preparation of the raw product (unitless); AmCs is the average annual concentration of 137Cs in foodstuff m for each year from 1966 to 1974 (Bq kg-1(L-1)).
for the list of foodstuffs) (kg (L) d-1); PFmCs is the processing factor that reflects the change in activity concentration of 137Cs resulting from the culinary preparation of the raw product (unitless); AmCs is the average annual concentration of 137Cs in foodstuff m for each year from 1966 to 1974 (Bq kg-1(L-1)). , 1969
, 1969 , 1971
, 1971 -1975
-1975 ) and in de-classified reports (SMSR, 1970
) and in de-classified reports (SMSR, 1970

 ; SMCB, 1975a,b, 1976a,b, 1978a,b).
; SMCB, 1975a,b, 1976a,b, 1978a,b).
Tableau 12 Archipelago-specific values of the behavioral factor, BFk (Drozdovitch et al., 2020b )
)
|
Age
|
Type of residence
|
Tahiti
|
Society Islands
|
Tuamotu archipelago
|
Gambier Islands
|
Marquesas Islands
|
Austral Islands
|
|
0-12 mo
|
Concrete house
|
0.30
|
0.31
|
0.35
|
0.30
|
0.30
|
0.31
|
|
Wooden (pinex) house
|
0.50
|
0.51
|
0.55
|
0.50
|
0.50
|
0.51
|
|
|
Bamboo house
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
|
|
Concrete apartment
|
0.10
|
0.12a
|
-
|
-
|
-
|
-
|
|
|
Unknownb
|
0.50
|
0.59
|
0.63
|
0.47
|
0.52
|
0.31
|
|
|
1-3.9 y
|
Concrete house
|
0.33
|
0.32
|
0.34
|
0.33
|
0.36
|
0.34
|
|
Wooden (pinex) house
|
0.53
|
0.52
|
0.54
|
0.53
|
0.56
|
0.54
|
|
|
Bamboo house
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
|
|
Concrete apartment
|
0.16
|
0.14a
|
-
|
-
|
-
|
-
|
|
|
Unknown
|
0.53
|
0.60
|
0.63
|
0.50
|
0.57
|
0.34
|
|
|
4-6.9 y
|
Concrete house
|
0.36
|
0.33
|
0.36
|
0.34
|
0.36
|
0.38
|
|
Wooden (pinex) house
|
0.56
|
0.53
|
0.56
|
0.54
|
0.56
|
0.58
|
|
|
Bamboo house
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
|
|
Concrete apartment
|
0.23
|
0.16a
|
-
|
-
|
-
|
-
|
|
|
Unknown
|
0.55
|
0.61
|
0.64
|
0.51
|
0.53
|
0.38
|
|
|
7-14.9 yc
|
Concrete house
|
0.33
|
0.33
|
0.34
|
0.33
|
0.33
|
0.31
|
|
Wooden (pinex) house
|
0.53
|
0.53
|
0.54
|
0.53
|
0.53
|
0.51
|
|
|
Bamboo house
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
|
|
Concrete apartment
|
0.15
|
0.15a
|
-
|
-
|
-
|
-
|
|
|
Unknown
|
0.52
|
0.60
|
0.63
|
0.50
|
0.50
|
0.31
|
|
|
7-14.9 yd
|
Concrete house
|
0.34
|
0.37
|
0.38
|
0.37
|
0.37
|
0.38
|
|
Wooden (pinex) house
|
0.54
|
0.57
|
0.58
|
0.57
|
0.57
|
0.58
|
|
|
Bamboo house
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
|
|
Concrete apartment
|
0.18
|
0.23a
|
-
|
-
|
-
|
-
|
|
|
Unknown
|
0.54
|
0.63
|
0.65
|
0.54
|
0.53
|
0.38
|
|
|
15-21 y
|
Concrete house
|
0.33
|
0.35
|
0.38
|
0.37
|
0.33
|
0.38
|
|
Wooden (pinex) house
|
0.53
|
0.55
|
0.58
|
0.57
|
0.53
|
0.58
|
|
|
Bamboo house
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
0.70
|
|
|
Concrete apartment
|
0.17
|
0.19a
|
-
|
-
|
-
|
-
|
|
|
Unknown
|
0.53
|
0.62
|
0.65
|
0.54
|
0.50
|
0.54
|
a Only in Raiatea.
b For unknown type of residence, behavior factor was estimated using archipelago-specific combination of type and construction material of residences according to Drozdovitch et al. (2019 ).
).
c During school days.
d During school vacation from 15 June to 15 August.
 ). For foodstuffs other than those listed above, the value of the processing factor was 1.0.
). For foodstuffs other than those listed above, the value of the processing factor was 1.0.Study subjects exposed in utero
 ); DCinh,ifetus(tg) is the thyroid dose coefficient to a fetus of gestational age tg at time TOA of the test under consideration, per unit of acute inhalation intake of radioiodine or tellurium isotopes by the mother (ICRP, 2001) (mGy Bq-1).
); DCinh,ifetus(tg) is the thyroid dose coefficient to a fetus of gestational age tg at time TOA of the test under consideration, per unit of acute inhalation intake of radioiodine or tellurium isotopes by the mother (ICRP, 2001) (mGy Bq-1). ), are given in Table 13
), are given in Table 13 . The consumption rate of foodstuff m by pregnant women used in this study, V*m,preg, was calculated from the focus-group data as:
. The consumption rate of foodstuff m by pregnant women used in this study, V*m,preg, was calculated from the focus-group data as:Breastfed study subjects
Tableau 13 Archipelago-specific fractions of consumers (Pcons,preg) and consumption ratesa of locally produced foodstuffs, Vm,preg (kg (L) d-1), by pregnant women (Drozdovitch et al., 2020b )
)
|
Foodstuff
|
Tahiti
|
Society Islands
|
Tuamotu archipelago
|
Gambier Islands
|
Marquesas Islands
|
Austral Islands
|
||||||
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
|
|
Fresh cow’s milk
|
0.30
|
0.15
|
0.30
|
0.15
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
Leafy vegetablesb
|
0.72
|
0.10
|
0.83
|
0.070
|
0.40
|
0.065
|
1.0
|
0.070
|
0.44
|
0.093
|
0.89
|
0.14
|
|
Fâfâ
|
0.66
|
0.074
|
0.50
|
0.060
|
-
|
-
|
0.43
|
0.14
|
-
|
-
|
0.89
|
0.20
|
|
Coco milk
|
0.74
|
0.29
|
0.67
|
0.19
|
1.0
|
0.36
|
1.0
|
0.31
|
0.56
|
0.11
|
0.78
|
0.16
|
|
Coco copra
|
0.85
|
0.077
|
0.75
|
0.060
|
0.93
|
0.10
|
0.71
|
0.056
|
1.0
|
0.31
|
0.78
|
0.11
|
|
Uru
|
0.77
|
0.20
|
0.83
|
0.090
|
0.93
|
0.47
|
1.0
|
0.21
|
1.0
|
0.090
|
0.78
|
0.18
|
|
Banana
|
0.89
|
0.23
|
0.67
|
0.16
|
0.93
|
0.18
|
1.0
|
0.44
|
0.89
|
0.45
|
1.0
|
0.36
|
|
Mango
|
0.91
|
0.41
|
0.75
|
0.56
|
-c
|
- c
|
1.0
|
0.33
|
0.89
|
0.61
|
0.67
|
0.58
|
|
Papaya
|
0.79
|
0.24
|
0.67
|
0.23
|
1.0
|
0.38
|
0.71
|
0.31
|
0.89
|
0.44
|
0.78
|
0.30
|
|
Manioc
|
0.60
|
0.10
|
0.83
|
0.074
|
-c
|
- c
|
1.0
|
0.13
|
0.78
|
0.10
|
0.89
|
0.20
|
|
Taro
|
0.91
|
0.13
|
0.83
|
0.078
|
-c
|
- c
|
-c
|
- c
|
0.56
|
0.036
|
1.0
|
0.35
|
|
Sweet potatoes
|
0.6
|
0.063
|
0.83
|
0.078
|
-c
|
- c
|
0.86
|
0.10
|
-c
|
- c
|
0.78
|
0.15
|
|
Poultry
|
0.74
|
0.070
|
0.75
|
0.064
|
0.87
|
0.068
|
1.0
|
0.15
|
0.78
|
0.19
|
0.78
|
0.074
|
|
Beef
|
0.51
|
0.036
|
0.58
|
0.031
|
-c
|
- c
|
0.57
|
0.044
|
0.78
|
0.055
|
0.33
|
0.041
|
|
Pork
|
0.38
|
0.049
|
0.42
|
0.050
|
0.87
|
0.052
|
0.71
|
0.069
|
0.78
|
0.16
|
0.56
|
0.045
|
|
Goat
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
0.78
|
0.065
|
-
|
-
|
|
Benitier
|
0.51
|
0.045
|
0.42
|
0.047
|
0.93
|
0.053
|
0.57
|
0.031
|
0.67
|
0.028
|
0.22
|
0.058
|
|
Fish (sea, lagoon)
|
0.98
|
0.23
|
0.83
|
0.27
|
1.0
|
0.50
|
1.0
|
0.24
|
0.67
|
0.22
|
1.0
|
0.29
|
a Consumption rates are provided for consumers only.
b Leafy vegetables including pota, watercress, spinach, lettuce.
c Foodstuff consumed but not locally produced.
 ) (mGy Bq-1).
) (mGy Bq-1). ) (mGy Bq-1); V*m,lact is the consumption rate of foodstuff m or drinking water by the lactating woman (kg (L) d-1).
) (mGy Bq-1); V*m,lact is the consumption rate of foodstuff m or drinking water by the lactating woman (kg (L) d-1).
Tableau 14 Archipelago-specific fractions of consumers (Pcons,lact) and consumption ratesa of locally produced foodstuffs, Vm,lact (kg (L) d-1), by lactating women (Drozdovitch et al., 2019 )
)
|
Foodstuff
|
Tahiti
|
Society Islands
|
Tuamotu archipelago
|
Gambier Islands
|
Marquesas Islands
|
Austral Islands
|
||||||
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
Pcons
|
Vm
|
|
|
Fresh cow’s milk
|
0.30
|
0.17
|
0.30
|
0.15
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
Leafy vegetablesb
|
0.70
|
0.10
|
0.75
|
0.068
|
0.40
|
0.065
|
1.0
|
0.070
|
0.44
|
0.093
|
0.89
|
0.14
|
|
Fâfâ
|
0.66
|
0.074
|
0.67
|
0.055
|
-
|
-
|
0.43
|
0.14
|
-
|
-
|
0.89
|
0.20
|
|
Coco milk
|
0.72
|
0.24
|
0.58
|
0.11
|
0.93
|
0.36
|
1.0
|
0.31
|
0.56
|
0.11
|
0.78
|
0.16
|
|
Coco copra
|
0.83
|
0.068
|
0.83
|
0.056
|
0.87
|
0.11
|
0.71
|
0.056
|
1.0
|
0.30
|
0.78
|
0.11
|
|
Uru
|
0.72
|
0.20
|
0.83
|
0.093
|
0.93
|
0.46
|
1.0
|
0.21
|
1.0
|
0.066
|
0.78
|
0.18
|
|
Banana
|
0.83
|
0.22
|
0.75
|
0.17
|
0.87
|
0.18
|
1.0
|
0.44
|
0.89
|
0.37
|
1.0
|
0.36
|
|
Mango
|
0.87
|
0.40
|
0.75
|
0.46
|
-c
|
- c
|
1.0
|
0.33
|
0.78
|
0.59
|
0.67
|
0.58
|
|
Papaya
|
0.77
|
0.23
|
0.75
|
0.28
|
1.0
|
0.38
|
0.71
|
0.31
|
0.89
|
0.38
|
0.78
|
0.30
|
|
Manioc
|
0.64
|
0.066
|
0.83
|
0.074
|
-c
|
- c
|
1.0
|
0.13
|
0.78
|
0.10
|
0.89
|
0.20
|
|
Taro
|
0.96
|
0.12
|
0.83
|
0.074
|
-c
|
- c
|
-c
|
- c
|
0.56
|
0.036
|
1.0
|
0.35
|
|
Sweet potatoes
|
0.62
|
0.061
|
0.83
|
0.074
|
-c
|
- c
|
0.86
|
0.10
|
-c
|
- c
|
0.78
|
0.15
|
|
Poultry
|
0.72
|
0.072
|
0.75
|
0.067
|
0.93
|
0.071
|
1.0
|
0.15
|
0.78
|
0.19
|
0.78
|
0.074
|
|
Beef
|
0.51
|
0.036
|
0.58
|
0.027
|
-c
|
- c
|
0.57
|
0.044
|
0.78
|
0.055
|
0.33
|
0.041
|
|
Pork
|
0.38
|
0.049
|
0.42
|
0.050
|
0.80
|
0.056
|
0.71
|
0.069
|
0.78
|
0.16
|
0.56
|
0.045
|
|
Goat
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
0.78
|
0.065
|
-
|
-
|
|
Benitier
|
0.49
|
0.039
|
0.42
|
0.051
|
0.87
|
0.054
|
0.57
|
0.031
|
0.67
|
0.028
|
0.22
|
0.058
|
|
Fish (sea, lagoon)
|
1.0
|
0.25
|
0.83
|
0.30
|
1.0
|
0.50
|
1.0
|
0.24
|
0.67
|
0.22
|
1.0
|
0.29
|
a Consumption rates are provided for consumers only.
b Leafy vegetables including pota, watercress, spinach, lettuce.
c Foodstuff consumed but not locally produced.
 ), are given in Table 14
), are given in Table 14 . By analogy with pregnant women, the consumption of foodstuff m by lactating women used in this study, V*m,lact, was calculated from the focus-group data as:
. By analogy with pregnant women, the consumption of foodstuff m by lactating women used in this study, V*m,lact, was calculated from the focus-group data as:Individual thyroid dose estimates
 . The average thyroid dose due to all exposure pathways combined was estimated to be 4.7 mGy (range: 0.014 mGy to 36 mGy), including: (1) 3.5 mGy (range: 0.002 mGy to 27 mGy) due to intake of 131I, (2) 0.75 mGy (range: 0.001 mGy to 14 mGy) due to intake of short-lived iodine isotopes (132I, 133I, 135I) and 132Te, (3) 0.41 mGy (range: 0.005 mGy to 5.8 mGy) from external irradiation, and (4) 0.08 mGy (range: ~0 mGy to 0.94 mGy) from ingestion of 137Cs.
. The average thyroid dose due to all exposure pathways combined was estimated to be 4.7 mGy (range: 0.014 mGy to 36 mGy), including: (1) 3.5 mGy (range: 0.002 mGy to 27 mGy) due to intake of 131I, (2) 0.75 mGy (range: 0.001 mGy to 14 mGy) due to intake of short-lived iodine isotopes (132I, 133I, 135I) and 132Te, (3) 0.41 mGy (range: 0.005 mGy to 5.8 mGy) from external irradiation, and (4) 0.08 mGy (range: ~0 mGy to 0.94 mGy) from ingestion of 137Cs.
Tableau 15 Thyroid doses reconstructed for the study subjects from different exposure pathways (Drozdovitch et al., 2020b )
)
|
Thyroid dose (mGy)
|
|||
|
Exposure pathway
|
Min
|
Mean
|
Max
|
|
Intake of 131I
|
0.002
|
3.5
|
27
|
|
Intake of short-lived 132I, 133I, 135I, and 132Te
|
0.001
|
0.75
|
14
|
|
External exposure
|
0.005
|
0.41
|
5.8
|
|
134,137Cs ingestion
|
3.4 × 10-5
|
0.08
|
0.94
|
|
All exposure pathways
|
0.014
|
4.7
|
36
|
 compares the contributions of each exposure pathway to the total thyroid dose of the study subjects by archipelago: intake of 131I and short-lived radioiodine isotopes (132I, 133I, 135I) and 132Te with (i) inhaled air, (ii) cow’s milk, (iii) leafy vegetables, and (iv) drinking water, in addition to (v) external irradiation, and (vi) ingestion of 137Cs with foodstuffs. The residents of the Society Islands received thyroid doses, mainly, from consumption of leafy vegetables and fresh cow’s milk with minor contributions from other pathways. The large contribution to the thyroid dose from the consumption of leafy vegetables was found for the study subjects who resided in the Marquesas Islands and, especially, in the Austral Islands. For the Austral Islands, the relatively high consumption rates of leafy vegetables were reported both by the study subjects during their personal interview and during the focus-group study (Drozdovitch et al., 2019
compares the contributions of each exposure pathway to the total thyroid dose of the study subjects by archipelago: intake of 131I and short-lived radioiodine isotopes (132I, 133I, 135I) and 132Te with (i) inhaled air, (ii) cow’s milk, (iii) leafy vegetables, and (iv) drinking water, in addition to (v) external irradiation, and (vi) ingestion of 137Cs with foodstuffs. The residents of the Society Islands received thyroid doses, mainly, from consumption of leafy vegetables and fresh cow’s milk with minor contributions from other pathways. The large contribution to the thyroid dose from the consumption of leafy vegetables was found for the study subjects who resided in the Marquesas Islands and, especially, in the Austral Islands. For the Austral Islands, the relatively high consumption rates of leafy vegetables were reported both by the study subjects during their personal interview and during the focus-group study (Drozdovitch et al., 2019 ). For the study subjects who resided in Tuamotu archipelago, drinking of rainwater followed by consumption of leafy vegetables and external irradiation were found to be the major contributors to the thyroid dose.
). For the study subjects who resided in Tuamotu archipelago, drinking of rainwater followed by consumption of leafy vegetables and external irradiation were found to be the major contributors to the thyroid dose. presents the annual thyroid doses received by the study subjects during the period of atmospheric testing in French Polynesia (1966-1974). The lowest thyroid doses were received in 1968, 1970 and 1972. Atmospheric tests were not conducted in 1969, but exposure to fallout occurred due to ingestion of 137Cs with locally produced food. The highest thyroid dose of 30 mGy is estimated to have resulted from the test Aldébaran in 1966 by a study subject who resided in the Gambier Islands. In 1967, thyroid doses of up to 6-7 mGy were received by study subjects who resided in Tahiti, Reao, and Takakoto (Tuamotu), but fallout was deposited rather uniformly throughout French Polynesia. In 1973, the maximal thyroid dose of 23 mGy was received after test Pallas by a study subject who resided in Rapa (Austral Islands). The major exposure in 1974 occurred after test Centaure in Tahiti and other Society Islands with a maximal thyroid dose among the study subjects of 21 mGy.
presents the annual thyroid doses received by the study subjects during the period of atmospheric testing in French Polynesia (1966-1974). The lowest thyroid doses were received in 1968, 1970 and 1972. Atmospheric tests were not conducted in 1969, but exposure to fallout occurred due to ingestion of 137Cs with locally produced food. The highest thyroid dose of 30 mGy is estimated to have resulted from the test Aldébaran in 1966 by a study subject who resided in the Gambier Islands. In 1967, thyroid doses of up to 6-7 mGy were received by study subjects who resided in Tahiti, Reao, and Takakoto (Tuamotu), but fallout was deposited rather uniformly throughout French Polynesia. In 1973, the maximal thyroid dose of 23 mGy was received after test Pallas by a study subject who resided in Rapa (Austral Islands). The major exposure in 1974 occurred after test Centaure in Tahiti and other Society Islands with a maximal thyroid dose among the study subjects of 21 mGy.
Tableau 16 Thyroid doses received by the study subjectsa in each year of the atmospheric testing period in French Polynesia (1966-1974) (Drozdovitch et al., 2020b )
)
|
Year
|
Thyroid dose (mGy)
|
||
|
Min
|
Mean
|
Max
|
|
|
1966
|
0.006
|
0.62
|
30
|
|
1967
|
0.018
|
1.0
|
7.2
|
|
1968
|
0.004
|
0.29
|
1.7
|
|
1969 b
|
1.1 × 10-4
|
0.011
|
0.079
|
|
1970
|
0.007
|
0.15
|
1.0
|
|
1971
|
0.002
|
0.37
|
4.2
|
|
1972
|
2.6 × 10-4
|
0.058
|
0.41
|
|
1973
|
0.001
|
0.33
|
23
|
|
1974
|
5.4 × 10-4
|
2.5
|
21
|
|
All years
|
0.014
|
4.7
|
36
|
a Only individuals who resided at given year in French Polynesia and were exposed to fallout.
b Exposure due to ingestion of 137Cs in locally-produced foodstuffs.
 compares the mean thyroid doses among study subjects received after different atmospheric nuclear tests that contributed substantially to the local deposition in French Polynesia. The highest mean dose among the study subjects was observed after the test Centaure in 1974. The highest thyroid doses after different atmospheric nuclear tests are also compared in Table 17
compares the mean thyroid doses among study subjects received after different atmospheric nuclear tests that contributed substantially to the local deposition in French Polynesia. The highest mean dose among the study subjects was observed after the test Centaure in 1974. The highest thyroid doses after different atmospheric nuclear tests are also compared in Table 17 . The highest individual dose was estimated for a subject who resided in Tahiti after the test Centaure in 1974.
. The highest individual dose was estimated for a subject who resided in Tahiti after the test Centaure in 1974. , 2020b
, 2020b ) were compared with results obtained earlier (UNSCEAR, 1977
) were compared with results obtained earlier (UNSCEAR, 1977 ; Bourges, 1997
; Bourges, 1997 ). The consumption rates of milk (0.7 L d-1), leafy vegetables (0.1 kg d-1) and drinking water (2 L d-1) that were used by UNSCEAR (1977
). The consumption rates of milk (0.7 L d-1), leafy vegetables (0.1 kg d-1) and drinking water (2 L d-1) that were used by UNSCEAR (1977 ) and by Bourges (1997
) and by Bourges (1997 ) were also applied by Drozdovitch et al. (2008
) were also applied by Drozdovitch et al. (2008 ). Table 18
). Table 18 compares the dose estimates published by UNSCEAR (1977
compares the dose estimates published by UNSCEAR (1977 ) and by Bourges (1997
) and by Bourges (1997 ) with the average doses reconstructed based on input data and methodology of Drozdovitch et al. (2008
) with the average doses reconstructed based on input data and methodology of Drozdovitch et al. (2008 ). In general, the agreement between the dose estimates obtained in the different studies is reasonable. Results of measurements of 131I concentration in cow’s milk were used for dose reconstruction in all studies; the differences in the dose estimates may be due to differences in data processing or in the values of the parameters used in the models.
). In general, the agreement between the dose estimates obtained in the different studies is reasonable. Results of measurements of 131I concentration in cow’s milk were used for dose reconstruction in all studies; the differences in the dose estimates may be due to differences in data processing or in the values of the parameters used in the models.
Tableau 17 Estimated thyroid dosesa,b received by the study subjects from each of the 41 atmospheric nuclear tests (Drozdovitch et al., 2020b )
)
|
Test
|
Date of test
(dd/mm/yyyy)
|
Thyroid dose (mGy)
|
||
|
Min
|
Mean
|
Max
|
||
|
Aldébaran
|
02/07/1966
|
0.002
|
0.22
|
30
|
|
Tamouré
|
19/07/1966
|
4.8 × 10-4
|
0.018
|
0.19
|
|
Bételgeuse
|
11/09/1966
|
0.002
|
0.089
|
0.55
|
|
Rigel
|
24/09/1966
|
3.5 × 10-4
|
0.012
|
0.29
|
|
Sirius
|
04/10/1966
|
0.006
|
0.27
|
3.1
|
|
Altaïr
|
05/06/1967
|
0.002
|
0.11
|
0.68
|
|
Antarès
|
27/06/1967
|
7.4 × 10-4
|
0.035
|
0.24
|
|
Arcturus
|
02/07/1967
|
0.014
|
0.84
|
6.7
|
|
Capella
|
07/07/1968
|
1.8 × 10-4
|
0.035
|
0.26
|
|
Castor
|
15/07/1968
|
0.001
|
0.051
|
0.29
|
|
Pollux
|
03/08/1968
|
0.004
|
0.15
|
0.92
|
|
Canopus
|
24/08/1968
|
9.8 × 10-4
|
0.026
|
0.28
|
|
Procyon
|
08/09/1968
|
5.8 × 10-4
|
0.025
|
0.17
|
|
Andromède
|
15/05/1970
|
1.8 × 10-4
|
0.016
|
0.13
|
|
Cassiopée
|
22/05/1970
|
3.6 × 10-4
|
0.011
|
0.081
|
|
Dragon
|
30/05/1970
|
6.5 × 10-5
|
0.004
|
0.19
|
|
Eridan
|
24/06/1970
|
2.2 × 10-4
|
0.008
|
0.16
|
|
Licorne
|
03/07/1970
|
0.001
|
0.030
|
0.35
|
|
Pégase
|
27/07/1970
|
3.7 × 10-4
|
0.022
|
0.14
|
|
Orion
|
02/08/1970
|
0.001
|
0.027
|
0.18
|
|
Toucan
|
06/08/1970
|
7.4 × 10-4
|
0.031
|
0.46
|
|
Dioné
|
05/06/1971
|
8.6 × 10-4
|
0.012
|
0.34
|
|
Encelade
|
12/06/1971
|
0.003
|
0.28
|
2.3
|
|
Japet
|
04/07/1971
|
3.7 × 10-4
|
0.040
|
0.47
|
|
Phoebé
|
08/08/1971
|
8.3 × 10-5
|
0.023
|
3.6
|
|
Rhéa
|
14/08/1971
|
8.2 × 10-5
|
0.014
|
0.32
|
|
Umbriel
|
25/06/1972
|
6.8 × 10-4
|
0.021
|
0.19
|
|
Titania
|
30/06/1972
|
3.7 × 10-4
|
0.014
|
0.24
|
|
Obéron
|
27/07/1972
|
1.4 × 10-4
|
0.016
|
0.11
|
|
Euterpe
|
21/07/1973
|
2.7 × 10-4
|
0.002
|
0.045
|
|
Melpomène
|
28/07/1973
|
4.9 × 10-4
|
0.002
|
0.005
|
|
Pallas
|
18/08/1973
|
8.6 × 10-4
|
0.30
|
23
|
|
Parthénope
|
24/08/1973
|
5.1 × 10-4
|
0.029
|
0.37
|
|
Tamara
|
28/08/1973
|
0.001
|
0.011
|
0.12
|
|
Capricorne
|
16/06/1974
|
5.9 × 10-4
|
0.009
|
0.064
|
|
Gémeaux
|
07/07/1974
|
1.0 × 10-4
|
0.003
|
0.045
|
|
Centaure
|
17/07/1974
|
4.1 × 10-4
|
2.4
|
20
|
|
Maquis
|
25/07/1974
|
2.5 × 10-4
|
0.015
|
0.11
|
|
Scorpion
|
15/08/1974
|
5.3 × 10-5
|
0.060
|
0.51
|
|
Taureau
|
24/08/1974
|
2.7 × 10-4
|
0.095
|
0.85
|
|
Verseau
|
14/10/1974
|
1.3 × 10-4
|
0.012
|
0.13
|
a Only individuals who were exposed to fallout from given test.
b Do not include thyroid dose due to 137Cs ingestion as 137Cs activity in foodstuffs cannot be attributed to specific tests.
Tableau 18 Comparison of dose estimates: UNSCEAR (1977 ), Bourges (1997
), Bourges (1997 ), and Drozdovitch et al. (2008
), and Drozdovitch et al. (2008 )
)
|
Test
|
Date
|
Location
|
Exposure pathway
or ingestion of
| |||
|
1967
|
Tahiti
|
Milk
|
0.6
|
-
|
0.5
|
|
|
1968
|
Tahiti
|
Milk
|
0.6
|
-
|
0.5
|
|
|
1970
|
Tahiti
|
Milk
|
1.3
|
-
|
1.5
|
|
|
1971
|
Tahiti
|
Milk
|
2.1
|
-
|
1.7
|
|
|
1972
|
Tahiti
|
Milk
|
0.12
|
-
|
0.11
|
|
|
1973
|
Tahiti
|
Milk
|
1.3
|
-
|
1.3
|
|
|
1974
|
Tahiti
|
Milk
|
6.8
|
-
|
6.8
|
|
|
Aldébaran
|
02/07/66
|
Gambier
|
External
|
-
|
3.4
|
3.9
|
|
Arcturus
|
02/07/67
|
Tureia
|
External
|
-
|
0.7
|
0.4
|
|
Encelade
|
12/06/71
|
Tureia
|
External
|
-
|
0.9
|
1.0
|
|
Phoebé
|
08/08/71
|
Gambier
|
External
|
-
|
0.9
|
0.4
|
|
Centaure
|
17/07/74
|
Tahiti
|
External
|
-
|
0.6
|
0.4
|
|
Aldébaran
|
02/07/66
|
Gambier
|
Inhalation
|
-
|
0.2
|
0.4
|
|
Arcturus
|
02/07/67
|
Tureia
|
Inhalation
|
-
|
0.023
|
0.035
|
|
Encelade
|
12/06/71
|
Tureia
|
Inhalation
|
-
|
0.003
|
0.03
|
|
Phoebé
|
08/08/71
|
Gambier
|
Inhalation
|
-
|
0.002
|
0.04
|
|
Centaure
|
17/07/74
|
Tahiti
|
Inhalation
|
-
|
0.08
|
0.01
|
|
Aldébaran
|
02/07/66
|
Gambier
|
Leafy vegetables
|
-
|
1.2
|
1.9
|
|
Phoebé
|
08/08/71
|
Gambier
|
Leafy vegetables
|
-
|
0.2
|
0.3
|
|
Centaure
|
17/07/74
|
Tahiti
|
Leafy vegetables
|
-
|
0.04
|
0.08
|
|
Aldébaran
|
02/07/66
|
Gambier
|
Drinking water
|
-
|
0.14
|
0.13
|
|
Phoebé
|
08/08/71
|
Gambier
|
Drinking water
|
-
|
0.035
|
0.013
|
|
Centaure
|
17/07/74
|
Tahiti
|
Milk
|
-
|
0.3a
|
0.4
|
a Effective dose for 1-y old infants.
Uncertainties in thyroid dose estimates
 ). For islands and atolls where measurements were not performed, procedures of interpolation and extrapolation resulted in uncertainties in the estimated deposition densities within a factor of 2 to 3 around the best estimate. In addition, the results obtained for atmospheric nuclear weapons tests conducted at the Nevada test site in the USA were used to estimate the deposition densities of specific radionuclides (Hicks, 1981
). For islands and atolls where measurements were not performed, procedures of interpolation and extrapolation resulted in uncertainties in the estimated deposition densities within a factor of 2 to 3 around the best estimate. In addition, the results obtained for atmospheric nuclear weapons tests conducted at the Nevada test site in the USA were used to estimate the deposition densities of specific radionuclides (Hicks, 1981 ) because the corresponding data for the tests conducted in French Polynesia were not found in the open literature.
) because the corresponding data for the tests conducted in French Polynesia were not found in the open literature. ) reference thyroid-mass value of 12 g for the same age group (15 years, according to the ICRP definition). We used in this study a thyroidal uptake of 30% and the age-dependent values of the thyroid mass recommended by ICRP (ICRP, 2002
) reference thyroid-mass value of 12 g for the same age group (15 years, according to the ICRP definition). We used in this study a thyroidal uptake of 30% and the age-dependent values of the thyroid mass recommended by ICRP (ICRP, 2002 ).
). ) and is characterized by low reproducibility and validity if recollections exceeding 10 years (Maruti et al., 2005
) and is characterized by low reproducibility and validity if recollections exceeding 10 years (Maruti et al., 2005 ). For example, as information on the locations of the schools attended by the study subjects aged 7 to 14 was not available, it was assumed that the schools were in the islands / atolls of residence.
). For example, as information on the locations of the schools attended by the study subjects aged 7 to 14 was not available, it was assumed that the schools were in the islands / atolls of residence. ), it was subjectively estimated that the overall uncertainties of the thyroid doses in this study are characterized, on average, by a geometric standard deviation of 2.5 to 3.0.
), it was subjectively estimated that the overall uncertainties of the thyroid doses in this study are characterized, on average, by a geometric standard deviation of 2.5 to 3.0.Conclusions
Références