| dc.contributor.author | Saumon, G | fr_FR |
| dc.date.accessioned | 2012-08-23T13:57:01Z | |
| dc.date.available | 2012-08-23T13:57:01Z | |
| dc.date.issued | 1999 | fr_FR |
| dc.identifier.citation | Saumon, G, L'épithélium alvéolaire lors des oedèmes pulmonaires., Med Sci (Paris), 1999, Vol. 15, N° 6-7; p.778-87 | fr_FR |
| dc.identifier.issn | 1958-5381 | fr_FR |
| dc.identifier.uri | http://hdl.handle.net/10608/1434 | |
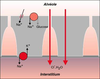
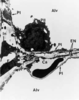
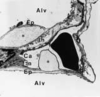
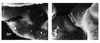
| dc.description.abstract | Lorsque la pression intracapillaire pulmonaire est tres elevee, des breches a distance des jonctions cellulaires ont pu etre observees, attribuees a des ruptures mecaniques du tissu. Celles-ci pourraient, en fait, refleter une reponse adaptative cellulaire plutot qu' une blessure mecanique. Le fluide de l' oedeme fait irruption dans l' alveole directement a travers la barriere epitheliale, s' accumulant d' abord dans les zones ou la pression est la plus basse. Les cellules epitheliales de type I se detachent par endroits de la lame basale et leur membrane plasmique forme d' enormes expansions pour confiner le liquide de l' oedeme et retarder l' entree de proteines dans les alveoles, preservant ainsi la fonction du surfactant. La resorption de l' oedeme met en jeu les transports ioniques actifs des cellules epitheliales, sous le controle d' agonistes <beta>-adrenergiques, de cytokines et de facteurs de croissance. La reabsorption liquidienne pourrait se faire au travers d' aquaporines. Le retour a la normale des proprietes de l' epithelium alveolaire est la cle de la resolution de l' oedeme. | fr |
| dc.description.abstract | The specific role of alveolar epithelium during the constitution and the resolution of alveolar edema has been the subject of numerous studies during the past decade that have conducted to complete reappraisal. Three notions that received general agreement have been reconsidered: the absence of lesions of the alveolo-capillary barrier during hemodynamic edema, the retrograde pathway taken by the edema fluid to flood alveoli and the role of Starling forces in governing alveolar liquid resorption. Extensive electron microscopy studies have shown that large increases in transmural capillary pressure may completely disrupt the alveolo-capillary barrier, a phenomenon called "stress failure". Stress failure may explain the occurrence of alveolar haemorrhage during heavy exercise in thoroughbred horses and the microvascular permeability alterations observed in some forms of pulmonary edema such as that produced by high volume ventilation. Recent works suggest that stress failure may reflect apreservative cellular response rather than a direct mechanical injury. The way alveolar edema develops has been clarified using a refined histological preparation. Edema fluid appears to make irruption into airspaces directly through the alveolar epithelial barrier, first accumulating in zones of low pressure such as the alveolar corners before completely filling distal airspaces. Alveolar epithelial type I cells display considerable plasticity during this transfer of liquid between the interstitial and alveolar compartments: epithelial type I cells detach by places from their basement membrane; their plasma membrane is deformed by huge expansions that confine edema fluid. This may be a way to delay the irruption of plasma proteins in the alveolar-airway lumen and to preserve surfactant activity. The mechanisms of alveolar edema resolution have received considerable attention. Alveolar liquid is absorbed following active transepithelial sodium transport by alveolar cells. In most species, the main pathway taken by sodium to enter cells at the apical membrane is constituted by epithelial sodium channels. Inactivation of these channels in a knock-out mouse model results in respiratory distress and early death. Transepithelial sodium transport and the clearance of alveolar fluid is positively modulated by hormones such as beta-adrenergics, cytokines such as tumour necrosis factor-alpha and growth factors such as keratinocyte growth factor. Maintenance of alveolar epithelial barrier integrity is also a key factor. The efficiency of active transepithelial sodium transport in driving water depends on the preservation of the epithelium barrier properties. There is evidence that the ability of the alveolar epithelium to concentrate proteins within airspaces is correlated with clinical improvement in patients suffering from various forms of pulmonary edema. | en |
| dc.language.iso | fr | fr_FR |
| dc.publisher | Masson Périodiques, Paris | fr_FR |
| dc.rights | Article en libre accès | fr |
| dc.rights | Médecine/Sciences - Inserm - SRMS | fr |
| dc.source | M/S. Médecine sciences [revue papier, ISSN : 0767-0974], 1999, Vol. 15, N° 6-7; p.778-87 | fr_FR |
| dc.title | L'épithélium alvéolaire lors des oedèmes pulmonaires. | fr |
| dc.title.alternative | Role of alveolar epithelium during pulmonary edema | fr_FR |
| dc.type | Article | fr_FR |
| dc.contributor.affiliation | Inserm U. 82, Faculte Xavier-Bichat, BP 416, 75877 Paris, France | - |
| dc.identifier.doi | 10.4267/10608/1434 | |