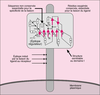
Topographie de la liaison intégrine-ligand.
Date
2001Auteur
Masson-Gadais, B
Huot, J
Lissitzky, JC
Voir/
Metadata
Afficher la notice complèteRésumé
Integrins are a family of heterodimeric transmembrane receptors constituted of 24 different pairs of alpha and beta subunits. They are recruted and activated in focal adhesions during cell-cell interaction or following cell adhesion to extracellular matrix. Integrins are actively involved in a bidirectional outside-in and inside-out signalling that underly cellular functions such as cell migration. Both alpha and beta subunits are implicated in the binding of integrins to their extracellular matrix ligands or counter-receptor on adjacent cells. The association (Kon) and dissociation (Koff) rates of integrins with/from their ligands, which defined affinity, is tightly regulated. However, the specific contribution of alpha and beta subunits of integrins in the association-dissociation with/from the ligand is unknown. We used a flux chamber to investigate the association/dissociation constants that characterize the interactions of alpha2beta1 integrins of keratinocytes adhering on type-1 collagen. We propose an hypothetic two-step model in which the alpha and beta subunits interact sequentially with two different regions of the ligand. The ligand first interacts with the alpha subunit which allosterically facilitates the binding of the ligand with the beta subunit. This stabilizes the ligand/integrin complex and reduces its dissociation rate. [References: 19]
Pour citer ce document
Masson-Gadais, B ; Huot, J ; Lissitzky, JC, Topographie de la liaison intégrine-ligand., Med Sci (Paris), 2001, Vol. 17, N° 2; p.206-10



