| dc.contributor.author | Boivin, P | fr_FR |
| dc.contributor.author | Lecomte, MC | fr_FR |
| dc.date.accessioned | 2012-07-11T08:41:40Z | |
| dc.date.available | 2012-07-11T08:41:40Z | |
| dc.date.issued | 1997 | fr_FR |
| dc.identifier.citation | Boivin, P ; Lecomte, MC, Les domaines homologues de la pleckstrine., Med Sci (Paris), 1997, Vol. 13, N° 5; p.639-46 | fr_FR |
| dc.identifier.issn | 1958-5381 | fr_FR |
| dc.identifier.uri | http://hdl.handle.net/10608/431 | |
| dc.description.abstract | La pleckstrine est le principal substrat de la protéine kinase
C dans les plaquettes. Elle possède deux segments homologues,
d’environ 100 acides aminés chacun, dénommés
domaines homologues de la pleckstrine (PH). Ces
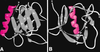
domaines ont une structure secondaire composée de sept
segments en plis β antiparallèles et d’une hélice α qui forment
un « sandwich » dans la structure tridimensionnelle.
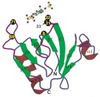
On retrouve des domaines homologues de la pleckstrine
dans un grand nombre de protéines dont le seul point commun
est qu’elles sont ou doivent être liées à la membrane
cellulaire pour exercer leur fonction : le domaine PH peut
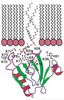
reconnaître particulièrement les phophoinositides membraniares
et les chaînes βγ des protéines G trimériques. Les
protéines à domaine PH peuvent aussi recruter d’autres
protéines cytosoliques qui ne sont actives que liées à la
membrane ou rapprochées de leurs ligands. De très nombreuses
protéines impliquées dans la transmission du signal
portent un domaine PH souvent associé aux domaines
homologues de Dbl ou aux domaines SH2 et SH3, ce qui
évoque la possibilité d’une coopération fonctionnelle. | fr |
| dc.description.abstract | Pleckstrin is the major substrate of protein kinase C in platelets. Expression cloning of pleckstrin yielded a cDNA encoding a protein with two internal repeats of about 120 aminoacids located at the N and C terminal extremities of the molecule. Database screening led to discover the presence of domains homologous to the pleckstrin repeats in more than 90 proteins devoid of common physiological function such as serine-threonine or tyrosine protein kinases, phospholipases, membrane and cytoskeletal proteins and many signaling proteins including activators and inhibitors of small G-protein. Some of them, especially the signaling proteins contain other homology domains such as SH2, SH3 and Dbl. All PH domains share a common tridimensional structure characterized by a seven-stranded anti-parallel beta-sheet and a strong bend forming an orthogonal sandwich which is closed by a C-terminal alpha-helix. The PH structures differ most in the loop regions between beta strands where more or less large insertions may be present. All PH domains contain a positively charged region towards the N-terminal of the domain. Most of the PH-containing proteins should be bound or approached to the cell membrane for assuming their function. The PH domains can be bound to the membrane through interaction of the positively charged region with the negatively charged phosphoinositides. Phospholipase Cdelta 1 (PLC delta1) binds its membrane substrate (PdIns 4,5)P2 through its PH domain with high specificity. Phosphoinositides are the main ligands of the PH domains. However C-terminal region of some PH domains binds the gamma component of the trimeric G proteins. PH Domains could be anchored to the membrane by both phosphoinositols and Ggamma proteins. Very probably other as yet unidentified ligands interact with the PH domains. Several different mutations in the PH domain of the Bruton tyrosine kinase are responsible of some cases of X-linked agammaglobulinemia in man and mice. At the present day they are the sole PH domain mutations associated with diseased state. [References: 41] | en |
| dc.language.iso | fr | fr_FR |
| dc.publisher | Masson, Paris | fr_FR |
| dc.rights | Article en libre accès | fr |
| dc.rights | Médecine/Sciences - Inserm - SRMS | fr |
| dc.source | M/S. Médecine sciences [revue papier, ISSN : 0767-0974], 1997, Vol. 13, N° 5; p.639-46 | fr_FR |
| dc.title | Les domaines homologues de la pleckstrine. | fr |
| dc.title.alternative | Protein domains homologous to pleckstrin repeats | fr_FR |
| dc.type | Article | fr_FR |
| dc.contributor.affiliation | Inserm U. 409, Faculte de medecine Xavier-Bichat, 16, rue Henri-Huchard, 75018 Paris, France | - |
| dc.identifier.doi | 10.4267/10608/431 | |