Domaines fonctionnels de l'enveloppe du VIH-1 et anticorps neutralisants
Résumé
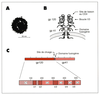
Two different epitopes located within the surface glycoprotein (gp120) of HIV1 induce the vast majority of neutralizing antibodies during natural infection. The first of them is a sequential epitope present in the third variable domain (V3 loop) of gp120 whereas the second one, highly conserved, is conformational. Neutralizing antibodies to V3 are <<type-specific>> since they are capable of blocking in vitro infection of a limited number of closely related HIV1 strains. Neutralizing antibodies directed to the conformational epitope are more cross-reactive capable of inhibiting the infection by a wider spectrum of HIV1 strains, and therefore named <<group-specific>>. These two domains of gp120 play major roles in the early steps of HIV1 cellular infection. The conformational epitope corresponds to the CD4 binding site and the V3 loop seems to interact with a membrane protease present at the surface of the target cells. Neutralizing antibodies would act as inhibitors of these two early molecular interactions necessary for virus entry. The two categories of antibodies act synergistically in neutralization assays in vitro. It appears that both epitopes must be included in any AIDS vaccine in order to induce theoretically efficient broadly neutralizing antibodies.
Pour citer ce document
Brand, D. ; Truong, C. ; Barin, F., Domaines fonctionnels de l'enveloppe du VIH-1 et anticorps neutralisants, Med Sci (Paris), 1994, Vol. 10, N° 4; p.417-424